Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals
- PMID: 34642411
- PMCID: PMC8511073
- DOI: 10.1038/s41598-021-99705-y
Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals
Abstract
Monocytes are thought to play an important role in host defence and pathogenesis of COVID-19. However, a comprehensive examination of monocyte numbers and function has not been performed longitudinally in acute and convalescent COVID-19. We examined the absolute counts of monocytes, the frequency of monocyte subsets, the plasma levels of monocyte activation markers using flowcytometry and ELISA in seven groups of COVID-19 individuals, classified based on days since RT-PCR confirmation of SARS-CoV2 infection. Our data shows that the absolute counts of total monocytes and the frequencies of intermediate and non-classical monocytes increases from Days 15-30 to Days 61-90 and plateau thereafter. In contrast, the frequency of classical monocytes decreases from Days 15-30 till Days 121-150. The plasma levels of sCD14, CRP, sCD163 and sTissue Factor (sTF)-all decrease from Days 15-30 till Days 151-180. COVID-19 patients with severe disease exhibit higher levels of monocyte counts and higher frequencies of classical monocytes and lower frequencies of intermediate and non-classical monocytes and elevated plasma levels of sCD14, CRP, sCD163 and sTF in comparison with mild disease. Thus, our study provides evidence of dynamic alterations in monocyte counts, subset frequencies and activation status in acute and convalescent COVID-19 individuals.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

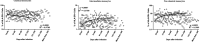


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

