Pharmacological treatment for bipolar mania: a systematic review and network meta-analysis of double-blind randomized controlled trials
- PMID: 34642461
- PMCID: PMC9054678
- DOI: 10.1038/s41380-021-01334-4
Pharmacological treatment for bipolar mania: a systematic review and network meta-analysis of double-blind randomized controlled trials
Abstract
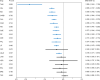
A systematic review and random-effects model network meta-analysis was conducted to compare the efficacy, acceptability, tolerability, and safety of pharmacological interventions for adults with acute bipolar mania. We searched PubMed, the Cochrane Library, and Embase databases for eligible studies published before March 14, 2021. Randomized controlled trials (RCTs) of oral medication monotherapy lasting ≥10 days in adults with mania were included, and studies that allowed the use of antipsychotics as a rescue medication during a trial were excluded. The primary outcomes were response to treatment (efficacy) and all-cause discontinuation (acceptability). The secondary outcomes were the improvement of mania symptoms and discontinuation due to inefficacy. Of the 79 eligible RCTs, 72 double-blind RCTs of 23 drugs and a placebo were included in the meta-analysis (mean study duration = 3.96 ± 2.39 weeks, n = 16442, mean age = 39.55 years, with 50.93% males). Compared with the placebo, aripiprazole, asenapine, carbamazepine, cariprazine, haloperidol, lithium, olanzapine, paliperidone, quetiapine, risperidone, tamoxifen, valproate, and ziprasidone outperformed response to treatment (N = 56, n = 14503); aripiprazole, olanzapine, quetiapine, and risperidone had lower all-cause discontinuation; however, topiramate had higher all-cause discontinuation (N = 70, n = 16324). Compared with the placebo, aripiprazole, asenapine, carbamazepine, cariprazine, haloperidol, lithium, olanzapine, paliperidone, quetiapine, risperidone, tamoxifen, valproate, and ziprasidone outperformed the improvement of mania symptoms (N = 61, n = 15466), and aripiprazole, asenapine, carbamazepine, cariprazine, haloperidol, lithium, olanzapine, paliperidone, quetiapine, risperidone, valproate, and ziprasidone had lower discontinuation due to inefficacy (N = 50, n = 14284). In conclusions, these antipsychotics, carbamazepine, lithium, tamoxifen, and valproate were effective for acute mania. However, only aripiprazole, olanzapine, quetiapine, and risperidone had better acceptability than the placebo.
© 2021. The Author(s).
Conflict of interest statement
The authors have declared that there are no conflicts of interest relating to the subject of this study. Interests from the past three years are as follows. TK received speaker’s honoraria from Sumitomo Dainippon, Otsuka, Eisai, Daiichi Sankyo, Janssen, Takeda, Kyowa, Kissei, Meiji, Pfizer, Mochida, Eli Lilly, MSD, Janssen, and Tanabe-Mitsubishi (Yoshitomi); as well as research grants from Eisai, the Japanese Ministry of Health, Labour and Welfare (H29-Seishin-Ippan-001, 19GC1012), Grant-in-Aid for Scientific Research (C), and Fujita Health University School of Medicine. TI has no conflicts of interest with any company. YM has received speaker’s honoraria from Dainippon Sumitomo, Janssen, Kyowa, Otsuka, Tanabe-Mitsubishi, and Yoshitomi. KS has received speaker’s honoraria from Eisai, Kissei, Meiji, Otsuka, and Torii; and has received a Fujita Health University School of Medicine Research Grant for Early-Career Scientists, as well as a Grant-in-Aid for Young Scientists (B). MO has received speaker’s honoraria from Meiji; and has received a Fujita Health University School of Medicine Research Grant for Early-Career Scientists, as well as a Grant-in-Aid for Young Scientists. IN has received speaker’s honoraria from Meiji, MSD, Janssen, and Torii. MH has received speaker’s honoraria from Dainippon Sumitomo and Otsuka. NI received speaker’s honoraria from Sumitomo Dainippon, Eli Lilly, Janssen, Meiji, Otsuka, Takeda, and Pfizer as well as research grants from Dainippon Sumitomo, Eisai, Otsuka, and Takeda; along with research grants from Daiichi Sankyo, Takeda, Dainippon Sumitomo Eisai, Meiji Tanabe-Mitsubishi, and Otsuka.
Figures



References
-
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97–170. doi: 10.1111/bdi.12609. - DOI - PMC - PubMed
-
- Fountoulakis KN, Yatham L, Grunze H, Vieta E, Young A, Blier P, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 2: review, grading of the evidence, and a precise algorithm. Int J Neuropsychopharmacol. 2017;20:121–79. - PMC - PubMed

