Dynamics of SARS-CoV-2-specific antibodies among COVID19 biobank donors in Argentina
- PMID: 34642643
- PMCID: PMC8494997
- DOI: 10.1016/j.heliyon.2021.e08140
Dynamics of SARS-CoV-2-specific antibodies among COVID19 biobank donors in Argentina
Abstract
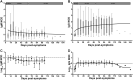
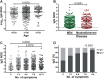
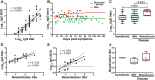
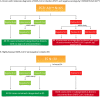
Biobanks are instrumental for accelerating research. Early in SARS-CoV-2 pandemic, the Argentinean Biobank of Infectious Diseases (BBEI) initiated the COVID19 collection and started its characterization. Blood samples from subjects with confirmed SARS-CoV-2 infection either admitted to health institutions or outpatients, were enrolled. Highly exposed seronegative individuals, were also enrolled. Longitudinal samples were obtained in a subset of donors, including persons who donated plasma for therapeutic purposes (plasma donors). SARS-CoV-2-specific IgM and IgG levels, IgG titers and IgG viral neutralization capacity were determined. Out of 825 donors, 57.1% were females and median age was 41 years (IQR 32-53 years). Donors were segregated as acute or convalescent donors, and mild versus moderate/severe disease donors. Seventy-eight percent showed seroconversion to SARS-CoV-2 specific antibodies. Specific IgM and IgG showed comparable positivity rates in acute donors. IgM detectability rate declined in convalescent donors while IgG detectability remained elevated in early (74,8%) and late (83%) convalescent donors. Among donors with follow-up samples, IgG levels seemed to decline more rapidly in plasma donors. IgG levels were higher with age, disease severity, number of symptoms, and more durable in moderate/severe disease donors. Levels and titers of anti-spike/RBD IgG strongly correlated with neutralization activity against WT virus. The BBEI-COVID19 collection serves a dual role in this SARS-CoV-2 global crisis. First, it feeds researchers and developers transferring samples and data to fuel research projects. Second, it generates highly needed local data to understand and frame the regional dynamics of the infection.
Keywords: Antibody response; Biobank; COVID-19; Convalescent plasma; SARS-CoV-2.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
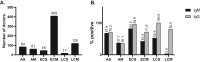


References
-
- World Health Organization . 2021. Coronavirus Disease (COVID-19) Situation Reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
-
- Ministerio de Salud de la Republica Argentina . 2021. COVID19 Daily Report.https://www.argentina.gob.ar/coronavirus/informes-diarios/reportes/abril...
LinkOut - more resources
Full Text Sources
Miscellaneous

