Cochlear Immune Response in Presbyacusis: a Focus on Dysregulation of Macrophage Activity
- PMID: 34642854
- PMCID: PMC8782976
- DOI: 10.1007/s10162-021-00819-x
Cochlear Immune Response in Presbyacusis: a Focus on Dysregulation of Macrophage Activity
Abstract
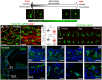
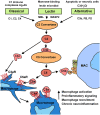
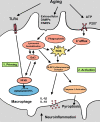
Age-related hearing loss, or presbyacusis, is a prominent chronic degenerative disorder that affects many older people. Based on presbyacusis pathology, the degeneration occurs in both sensory and non-sensory cells, along with changes in the cochlear microenvironment. The progression of age-related neurodegenerative diseases is associated with an altered microenvironment that reflects chronic inflammatory signaling. Under these conditions, resident and recruited immune cells, such as microglia/macrophages, have aberrant activity that contributes to chronic neuroinflammation and neural cell degeneration. Recently, researchers identified and characterized macrophages in human cochleae (including those from older donors). Along with the age-related changes in cochlear macrophages in animal models, these studies revealed that macrophages, an underappreciated group of immune cells, may play a critical role in maintaining the functional integrity of the cochlea. Although several studies deciphered the molecular mechanisms that regulate microglia/macrophage dysfunction in multiple neurodegenerative diseases, limited studies have assessed the mechanisms underlying macrophage dysfunction in aged cochleae. In this review, we highlight the age-related changes in cochlear macrophage activities in mouse and human temporal bones. We focus on how complement dysregulation and the nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 inflammasome could affect macrophage activity in the aged peripheral auditory system. By understanding the molecular mechanisms that underlie these regulatory systems, we may uncover therapeutic strategies to treat presbyacusis and other forms of sensorineural hearing loss.
Keywords: Auditory nerve; Complement system; Macrophages; NLRP3 inflammasome; Presbyacusis; Stria vascularis.
© 2021. Association for Research in Otolaryngology.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Stria Vascularis in Mice and Humans Is an Early Site of Age-Related Cochlear Degeneration, Macrophage Dysfunction, and Inflammation.J Neurosci. 2023 Jul 5;43(27):5057-5075. doi: 10.1523/JNEUROSCI.2234-22.2023. Epub 2023 Jun 2. J Neurosci. 2023. PMID: 37268417 Free PMC article.
-
The Stria Vascularis: Renewed Attention on a Key Player in Age-Related Hearing Loss.Int J Mol Sci. 2024 May 15;25(10):5391. doi: 10.3390/ijms25105391. Int J Mol Sci. 2024. PMID: 38791427 Free PMC article. Review.
-
Age-Related Changes in Immune Cells of the Human Cochlea.Front Neurol. 2019 Aug 16;10:895. doi: 10.3389/fneur.2019.00895. eCollection 2019. Front Neurol. 2019. PMID: 31474935 Free PMC article.
-
Quantitative analysis of cochlear sensory cells and neuronal elements in man.Acta Otolaryngol Suppl. 1990;470:71-9. doi: 10.3109/00016488909138359. Acta Otolaryngol Suppl. 1990. PMID: 2239237
-
Translational and interdisciplinary insights into presbyacusis: A multidimensional disease.Hear Res. 2021 Mar 15;402:108109. doi: 10.1016/j.heares.2020.108109. Epub 2020 Oct 31. Hear Res. 2021. PMID: 33189490 Free PMC article. Review.
Cited by
-
Cochlear inflammaging: cellular and molecular players of the innate and adaptive immune system in age-related hearing loss.Front Neurol. 2023 Nov 22;14:1308823. doi: 10.3389/fneur.2023.1308823. eCollection 2023. Front Neurol. 2023. PMID: 38073631 Free PMC article. Review.
-
Distribution of macrophages in the developing cochlea of the common marmoset, a primate model animal.Front Immunol. 2023 Aug 22;14:1229414. doi: 10.3389/fimmu.2023.1229414. eCollection 2023. Front Immunol. 2023. PMID: 37675123 Free PMC article.
-
Oxidative stress and inflammation cause auditory system damage via glial cell activation and dysregulated expression of gap junction proteins in an experimental model of styrene-induced oto/neurotoxicity.J Neuroinflammation. 2024 Jan 4;21(1):4. doi: 10.1186/s12974-023-02996-3. J Neuroinflammation. 2024. PMID: 38178142 Free PMC article.
-
Potential therapeutic role of SIRT1 in age- related hearing loss.Front Mol Neurosci. 2022 Sep 20;15:984292. doi: 10.3389/fnmol.2022.984292. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36204138 Free PMC article. Review.
-
Progress on mechanisms of age-related hearing loss.Front Neurosci. 2023 Aug 30;17:1253574. doi: 10.3389/fnins.2023.1253574. eCollection 2023. Front Neurosci. 2023. PMID: 37727326 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

