Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP)
- PMID: 34642875
- PMCID: PMC8508400
- DOI: 10.1007/s15010-021-01707-5
Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP)
Abstract
Purpose: Over the course of COVID-19 pandemic, evidence has accumulated that SARS-CoV-2 infections may affect multiple organs and have serious clinical sequelae, but on-site clinical examinations with non-hospitalized samples are rare. We, therefore, aimed to systematically assess the long-term health status of samples of hospitalized and non-hospitalized SARS-CoV-2 infected individuals from three regions in Germany.
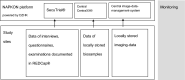
Methods: The present paper describes the COVIDOM-study within the population-based cohort platform (POP) which has been established under the auspices of the NAPKON infrastructure (German National Pandemic Cohort Network) of the national Network University Medicine (NUM). Comprehensive health assessments among SARS-CoV-2 infected individuals are conducted at least 6 months after the acute infection at the study sites Kiel, Würzburg and Berlin. Potential participants were identified and contacted via the local public health authorities, irrespective of the severity of the initial infection. A harmonized examination protocol has been implemented, consisting of detailed assessments of medical history, physical examinations, and the collection of multiple biosamples (e.g., serum, plasma, saliva, urine) for future analyses. In addition, patient-reported perception of the impact of local pandemic-related measures and infection on quality-of-life are obtained.
Results: As of July 2021, in total 6813 individuals infected in 2020 have been invited into the COVIDOM-study. Of these, about 36% wished to participate and 1295 have already been examined at least once.
Conclusion: NAPKON-POP COVIDOM-study complements other Long COVID studies assessing the long-term consequences of an infection with SARS-CoV-2 by providing detailed health data of population-based samples, including individuals with various degrees of disease severity.
Trial registration: Registered at the German registry for clinical studies (DRKS00023742).
Keywords: Internal medicine; Long COVID; Neurological; On-site examination; Population-based; Sars-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
Caroline Morbach reports research cooperation with the University of Würzburg and Tomtec Imaging Systems funded by a research grant from the Bavarian Ministry of Economic Affairs, Regional Development and Energy, Germany; advisory and speakers honoraria as well as travel grants from AKCEA, Alnylam, Amgen, Boehringer Ingelheim, EBR Systems Orion Pharma, Pfizer, SOBI, and Tomtec; principal investigator in trials sponsored by Alnylam and AstraZeneca; financial support from the interdisciplinary center for clinical research—IZKF Würzburg (advanced clinician-scientist program).
Figures
References
-
- Sahanic S, Sonnweber T, Pizzini A, Widmann G, Luger A, Aichner M, et al. Late Breaking Abstract - Persisting pulmonary impairment following severe SARS-CoV-2 infection, preliminary results from the CovILD study. Eur Respir J. 2020;56: Suppl. 64, 4143. 10.1183/13993003.congress-2020.4143
-
- Al Chikhanie Y, Verges S, Herengt F, Veale D. Late breaking abstract - the weekly recovery of physical capacities in COVID-19 patients during post-extubation pulmonary rehabilitation. Eur Respir J. 2020;56:938.
-
- WHO. WHO COVID-19 Explorer Geneva: World Health Organization; 2020 22/04/2021. https://worldhealthorg.shinyapps.io/covid/.
-
- Kurth F, Roennefarth M, Thibeault C, Corman V, Muller-Redetzky H, Mittermaier M, et al. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (Pa-COVID-19) Infection. 2020;48:619–626. doi: 10.1007/s15010-020-01464-x. - DOI - PMC - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous