Regulation of NANOG and SOX2 expression by activin A and a canonical WNT agonist in bovine embryonic stem cells and blastocysts
- PMID: 34643229
- PMCID: PMC8649639
- DOI: 10.1242/bio.058669
Regulation of NANOG and SOX2 expression by activin A and a canonical WNT agonist in bovine embryonic stem cells and blastocysts
Abstract
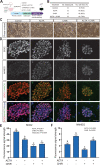
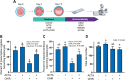
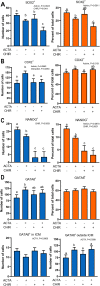
Bovine embryonic stem cells (ESC) have features associated with the primed pluripotent state including low expression of one of the core pluripotency transcription factors, NANOG. It has been reported that NANOG expression can be upregulated in porcine ESC by treatment with activin A and the WNT agonist CHIR99021. Accordingly, it was tested whether expression of NANOG and another pluripotency factor SOX2 could be stimulated by activin A and the WNT agonist CHIR99021. Immunoreactive NANOG and SOX2 were analyzed for bovine ESC lines derived under conditions in which activin A and CHIR99021 were added singly or in combination. Activin A enhanced NANOG expression but also reduced SOX2 expression. CHIR99021 depressed expression of both NANOG and SOX2. In a second experiment, activin A enhanced blastocyst development while CHIR99021 treatment impaired blastocyst formation and reduced number of blastomeres. Activin A treatment decreased blastomeres in the blastocyst that were positive for either NANOG or SOX2 but increased those that were CDX2+ and that were GATA6+ outside the inner cell mass. CHIR99021 reduced SOX2+ and NANOG+ blastomeres without affecting the number or percent of blastomeres that were CDX2+ and GATA6+. Results indicate activation of activin A signaling stimulates NANOG expression during self-renewal of bovine ESC but suppresses cells expressing pluripotency markers in the blastocyst and increases cells expressing CDX2. Actions of activin A to promote blastocyst development may involve its role in promoting trophectoderm formation. Furthermore, results demonstrate the negative role of canonical WNT signaling in cattle for pluripotency marker expression in ESC and in formation of the inner cell mass and epiblast during embryonic development. This article has an associated First Person interview with the first author of the paper.
Keywords: Activin; Bovine; Embryonic stem cells; Pluripotency; WNT signaling.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Bayerl, J., Ayyash, M., Shani, T., Manor, Y. S., Gafni, O., Massarwa, R., Kalma, Y., Aguilera-Castrejon, A., Zerbib, M., Amir, H.et al. (2021). Principles of signaling pathway modulation for enhancing human naive pluripotency induction. Cell Stem Cell 28, 1549-1565.e12. 10.1016/j.stem.2021.04.001 - DOI - PMC - PubMed

