Clinical Influenza Testing Practices in Hospitalized Children at United States Medical Centers, 2015-2018
- PMID: 34643241
- PMCID: PMC8794021
- DOI: 10.1093/jpids/piab096
Clinical Influenza Testing Practices in Hospitalized Children at United States Medical Centers, 2015-2018
Abstract
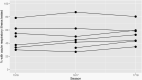
At nine US hospitals that enrolled children hospitalized with acute respiratory illness (ARI) during 2015-2016 through 2017-2018 influenza seasons, 50% of children with ARI received clinician-initiated testing for influenza and 35% of cases went undiagnosed due to lack of clinician-initiated testing. Marked heterogeneity in testing practice was observed across sites.
Keywords: RT-PCR; antigen test; hospitalized; influenza; testing.
© The Author(s) 2021. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Centers for Disease Control and Prevention. Flu & Young Children. Accessed July 20, 2021. https://www.cdc.gov/flu/highrisk/children.htm
-
- Campbell AP, Ogokeh C, Lively JY, et al. Vaccine effectiveness against pediatric influenza hospitalizations and emergency visits. Pediatrics 2020; 146(5):e20201368. - PubMed
MeSH terms
Grants and funding
- 5UL1TR002243-03/National Center for Advancing Translational Sciences Clinical Translational Science Award Program
- 5U01IP001035-02/CC/CDC HHS/United States
- U01 IP000972/IP/NCIRD CDC HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- U01IP001035/ACL/ACL HHS/United States
- U01 IP001158/IP/NCIRD CDC HHS/United States
- U01 IP001050/IP/NCIRD CDC HHS/United States
- U01 IP001157/IP/NCIRD CDC HHS/United States
- U01 IP001063/IP/NCIRD CDC HHS/United States
- U01 IP001156/IP/NCIRD CDC HHS/United States
- UL1TR001857/NH/NIH HHS/United States
- U01 IP001049/IP/NCIRD CDC HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

