Tau Protein Binding Modes in Alzheimer's Disease for Cationic Luminescent Ligands
- PMID: 34643404
- PMCID: PMC8558859
- DOI: 10.1021/acs.jpcb.1c06019
Tau Protein Binding Modes in Alzheimer's Disease for Cationic Luminescent Ligands
Abstract
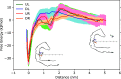
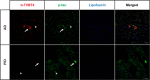
The bi-thiophene-vinylene-benzothiazole (bTVBT4) ligand developed for Alzheimer's disease (AD)-specific detection of amyloid tau has been studied by a combination of several theoretical methods and experimental spectroscopies. With reference to the cryo-EM tau structure of the tau protofilament ( Nature 2017, 547, 185), a periodic model system of the fibril was created, and the interactions between this fibril and bTVBT4 were studied with nonbiased molecular dynamics simulations. Several binding sites and binding modes were identified and analyzed, and the results for the most prevailing fibril site and ligand modes are presented. A key validation of the simulation work is provided by the favorable comparison of the theoretical and experimental absorption spectra of bTVBT4 in solution and bound to the protein. It is conclusively shown that the ligand-protein binding occurs at the hydrophobic pocket defined by the residues Ile360, Thr361, and His362. This binding site is not accessible in the Pick's disease (PiD) fold, and fluorescence imaging of bTVBT4-stained brain tissue samples from patients diagnosed with AD and PiD provides strong support for the proposed tau binding site.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


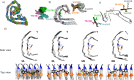

References
-
- Nguyen P. H.; Ramamoorthy A.; Sahoo B. R.; Zheng J.; Faller P.; Straub J. E.; Dominguez L.; Shea J.-E.; Dokholyan N. V.; De Simone A.; et al. Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem. Rev. 2021, 121, 2545–2647. 10.1021/acs.chemrev.0c01122. - DOI - PMC - PubMed
-
- Hanger D. P.; Byers H. L.; Wray S.; Leung K.-Y.; Saxton M. J.; Seereeram A.; Reynolds C. H.; Ward M. A.; Anderton B. H. Novel Phosphorylation Sites in Tau from Alzheimer Brain Support a Role for Casein Kinase 1 in Disease Pathogenesis. J. Biol. Chem. 2007, 282, 23645–23654. 10.1074/jbc.M703269200. - DOI - PubMed
-
- Smet-Nocca C.Tau Protein: Methods and Protocols; Smet-Nocca C., Ed.; Methods in Molecular Biology; Humana Press, 2017; Vol. 1523. https://www.springer.com/gp/book/9781493965960

