Routine, Cost-Effective SARS-CoV-2 Surveillance Testing Using Pooled Saliva Limits Viral Spread on a Residential College Campus
- PMID: 34643445
- PMCID: PMC8515933
- DOI: 10.1128/Spectrum.01089-21
Routine, Cost-Effective SARS-CoV-2 Surveillance Testing Using Pooled Saliva Limits Viral Spread on a Residential College Campus
Abstract
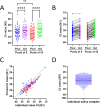
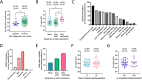
Routine testing for SARS-CoV-2 is rare for institutes of higher education due to prohibitive costs and supply chain delays. During spring 2021, we routinely tested all residential students 1 to 2 times per week using pooled, RNA-extraction-free, reverse transcription quantitative PCR (RT-qPCR) testing of saliva at a cost of $0.43/sample with same-day results. The limit of detection was 500 copies/ml on individual samples, and analysis indicates 1,000 and 2,500 copies/ml in pools of 5 and 10, respectively, which is orders of magnitude more sensitive than rapid antigen tests. Importantly, saliva testing flagged 83% of semester positives (43,884 tests administered) and was 95.6% concordant with nasopharyngeal diagnostic results (69.0% concordant on the first test when the nucleocapsid gene (N1) cycle threshold (CT) value was >30). Moreover, testing reduced weekly cases by 59.9% in the spring despite far looser restrictions, allowing for more normalcy while eliminating outbreaks. We also coupled our testing with a survey to clarify symptoms and transmissibility among college-age students. While only 8.5% remained asymptomatic throughout, symptoms were disparate and often cold-like (e.g., only 37.3% developed a fever), highlighting the difficulty with relying on symptom monitoring among this demographic. Based on reported symptom progression, we estimate that we removed 348 days of infectious individuals by routine testing. Interestingly, viral load (CT value) at the time of testing did not affect transmissibility (R2 = 0.0085), though those experiencing noticeable symptoms at the time of testing were more likely to spread the virus to close contacts (31.6% versus 14.3%). Together, our findings support routine testing for reducing the spread of SARS-CoV-2. Implementation of cost- and resource-efficient approaches should receive strong consideration in communities that lack herd immunity. IMPORTANCE This study highlights the utility of routine testing for SARS-CoV-2 using pooled saliva while maintaining high sensitivity of detection (under 2,500 copies/ml) and rapid turnaround of high volume (up to 930 samples in 8 h by two technicians and one quantitative PCR [qPCR] machine). This pooled approach allowed us to test all residential students 1 to 2 times per week on our college campus during the spring of 2021 and flagged 83% of our semester positives. Most students were asymptomatic or presented with symptoms mirroring common colds at the time of testing, allowing for removal of infectious individuals before they otherwise would have sought testing. To our knowledge, the total per-sample consumable cost of $0.43 is the lowest to date. With many communities still lagging in vaccination rates, routine testing that is cost-efficient highlights the capacity of the laboratory's role in controlling the spread of SARS-CoV-2.
Keywords: COVID-19; RT-qPCR; SARS-CoV-2; pooled; residential living; saliva; surveillance; surveillance studies.
Figures

References
-
- CDC. COVID data tracker. 2020. https://covid.cdc.gov/covid-data-tracker.
-
- Denny TN, Andrews L, Bonsignori M, Cavanaugh K, Datto MB, Deckard A, DeMarco CT, DeNaeyer N, Epling CA, Gurley T, Haase SB, Hallberg C, Harer J, Kneifel CL, Lee MJ, Louzao R, Moody MA, Moore Z, Polage CR, Puglin J, Spotts PH, Vaughn JA, Wolfe CR. 2020. Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections on a college campus: Duke University, Durham, North Carolina, August 2–October 11, 2020. MMWR Morb Mortal Wkly Rep 69:1743–1747. doi: 10.15585/mmwr.mm6946e1. - DOI - PMC - PubMed
-
- Rennert L, McMahan C, Kalbaugh CA, Yang Y, Lumsden B, Dean D, Pekarek L, Colenda CC. 2021. Surveillance-based informative testing for detection and containment of SARS-CoV-2 outbreaks on a public university campus: an observational and modelling study. Lancet Child Adolesc Health 5:428–436. doi: 10.1016/S2352-4642(21)00060-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

