The Bacterial Life Cycle in Textiles is Governed by Fiber Hydrophobicity
- PMID: 34643452
- PMCID: PMC8515937
- DOI: 10.1128/Spectrum.01185-21
The Bacterial Life Cycle in Textiles is Governed by Fiber Hydrophobicity
Abstract
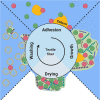
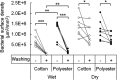
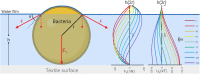
Colonization of textiles and subsequent metabolic degradation of sweat and sebum components by axillary skin bacteria cause the characteristic sweat malodor and discoloring of dirty clothes. Once inside the textile, the bacteria can form biofilms that are hard to remove by conventional washing. When the biofilm persists after washing, the textiles retain the sweat odor. To design biofilm removal and prevention strategies, the bacterial behavior needs to be understood in depth. Here, we aim to study the bacterial behavior in each of the four stages of the bacterial life cycle in textiles: adhesion, growth, drying, and washing. To accomplish this, we designed a novel in vitro model to mimic physiological sweating in cotton and polyester textiles, in which many of the parameters that influence bacterial behavior could be controlled. Due to the higher hydrophobicity, polyester adhered more bacteria and absorbed more sebum, the bacteria's primary nutrient source. Bacteria were therefore also more active in polyester textiles. However, polyester did not bind water as well as cotton. The increased water content of cotton allowed some species to retain a higher activity after the textile had dried. However, none of the textiles retained enough water upon drying to prevent the bacteria from adhering irreversibly to the textile fibers. This work demonstrates that bacterial colonization of textiles depends partially on the hydrophobic and hygroscopic properties of the textile material, indicating that it might be possible to direct bacterial behavior in a more favorable direction by modifying these surface properties. IMPORTANCE During sweating, bacteria from the skin enter the worn textile along with the sweat. Once inside the clothes, the bacteria produce sweat malodor and form colonies that are extremely hard to remove by washing. Over time, this leads to a decreasing textile quality and consumer comfort. To design prevention and removal mechanisms, we investigated the behavior of bacteria during the four stages of their life cycle in textiles: adhesion, growth, drying, and washing. The bacterial behavior in textiles during all four stages is found to be affected by the textile's ability to bind water and fat. The study indicates that sweat malodor and bacterial accumulation in textiles over time can be reduced by making the textiles more repellant to water and fat.
Keywords: biofilms; hydrophobicity; microbiology; skin; textile.
Figures





References
-
- Munk S, Münch P, Stahnke L, Adler-Nissen J, Schieberle P. 2000. Primary odorants of laundry soiled with sweat/sebum: influence of lipase on the odor profile. J Surfact Deterg 3:505–515. doi: 10.1007/s11743-000-0150-z. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

