Resolving the Near-Infrared Spectrum of Articular Cartilage
- PMID: 34643470
- PMCID: PMC8808936
- DOI: 10.1177/19476035211035417
Resolving the Near-Infrared Spectrum of Articular Cartilage
Abstract
Objective: Spectroscopic techniques, such as near-infrared (NIR) spectroscopy, are gaining significant research interest for characterizing connective tissues, particularly articular cartilage, because there is still a largely unmet need for rapid, accurate and objective methods for assessing tissue integrity in real-time during arthroscopic surgery. This study aims to identify the NIR spectral range that is optimal for characterizing cartilage integrity by (a) identifying the contribution of its major constituents (collagen and proteoglycans) to its overall spectrum using proxy constituent models and (b) determining constituent-specific spectral contributions that can be used for assessment of cartilage in its physiological state.
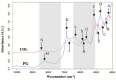
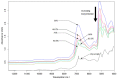
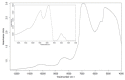
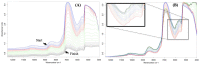
Design: The NIR spectra of cartilage matrix constituent models were measured and compared with specific molecular components of organic compounds in the NIR spectral range in order to identify their bands and molecular assignments. To verify the identified bands, spectra of the model compounds were compared with those of native cartilage. Since water obscures some bands in the NIR range, spectral measurements of the native cartilage were conducted under conditions of decreasing water content to amplify features of the solid matrix components. The identified spectral bands were then compared and examined in the resulting spectra of the intact cartilage samples.
Results: As water was progressively eliminated from cartilage, the specific contribution of the different matrix components was observed to correspond with those identified from the proxy cartilage component models.
Conclusion: Spectral peaks in the regions 5500 to 6250 cm-1 and 8100 to 8600 cm-1 were identified to be effective for characterizing cartilage proteoglycan and collagen contents, respectively.
Keywords: articular cartilage; collagen; matrix component models; near-infrared spectroscopy; proteoglycans.
Conflict of interest statement
Figures


References
-
- Buckwalter JA, Mankin HJ. Articular cartilage, part II: degeneration and osteoarthritis, repair, regeneration, and transplantation. J Bone Joint Surg. 1997;79-A:612-33. - PubMed
-
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. - PubMed
-
- Afara I, Prasadam I, Crawford R, Xiao Y, Oloyede A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthritis Cartilage. 2012;20:1367-73. - PubMed
-
- Afara IO, Prasadam I, Moody H, Crawford R, Xiao Y, Oloyede A. Near infrared spectroscopy for rapid determination of Mankin score components: a potential tool for quantitative characterization of articular cartilage at surgery. Arthroscopy. 2014;30:1146-55. - PubMed
-
- Spahn G, Plettenberg H, Nagel H, Kahl E, Klinger HM, Mückley T, et al.. Evaluation of cartilage defects with near-infrared spectroscopy (NIR): an ex vivo study. Med Eng Phys. 2008;30:285-92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

