Neutrophil-Derived Proteases Contribute to the Pathogenesis of Early Diabetic Retinopathy
- PMID: 34643662
- PMCID: PMC8525836
- DOI: 10.1167/iovs.62.13.7
Neutrophil-Derived Proteases Contribute to the Pathogenesis of Early Diabetic Retinopathy
Abstract
Purpose: Previous studies indicate that leukocytes, notably neutrophils, play a causal role in the capillary degeneration observed in diabetic retinopathy (DR), however, the mechanism by which they cause such degeneration is unknown. Neutrophil elastase (NE) is a protease released by neutrophils which participates in a variety of inflammatory diseases. In the present work, we investigated the potential involvement of NE in the development of early DR.
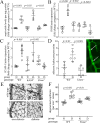
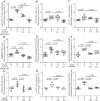
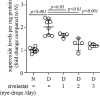
Methods: Experimental diabetes was induced in NE-deficient mice (Elane-/-), in mice treated daily with the NE inhibitor, sivelestat, and in mice overexpressing human alpha-1 antitrypsin (hAAT+). Mice were assessed for diabetes-induced retinal superoxide generation, inflammation, leukostasis, and capillary degeneration.
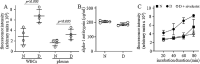
Results: In mice diabetic for 2 months, deletion of NE or selective inhibition of NE inhibited diabetes-induced retinal superoxide levels and inflammation, and inhibited leukocyte-mediated cytotoxicity of retinal endothelial cells. In mice diabetic for 8 months, genetic deletion of NE significantly inhibited diabetes-induced retinal capillary degeneration.
Conclusions: These results suggest that a protease released from neutrophils contributes to the development of DR, and that blocking NE activity could be a novel therapy to inhibit DR.
Conflict of interest statement
Disclosure:
Figures




References
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. .. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017; 128: 40–50. - PubMed
-
- Joussen AM, Poulaki V, Le ML, et al. .. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004; 18: 1450–1452. - PubMed
-
- Pavlovic D, Leteux C, Ovchinnikova T, Tsvetkov Y, Nifant'ev N, Feizi T. Chemically synthesized solid phase oligosaccharide probes for carbohydrate-binding receptors. Interactions of the E-, L- and P-selectins with sialyl-Le(x) and O-sulphated forms linked to biotin or to polyacrylamide. J Immunologic Methods. 2002; 264: 53–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

