Osimertinib in poor performance status patients with T790M-positive advanced non-small-cell lung cancer after progression of first- and second-generation EGFR-TKI treatments (NEJ032B)
- PMID: 34643820
- PMCID: PMC8732858
- DOI: 10.1007/s10147-021-02043-2
Osimertinib in poor performance status patients with T790M-positive advanced non-small-cell lung cancer after progression of first- and second-generation EGFR-TKI treatments (NEJ032B)
Erratum in
-
Correction to: Osimertinib in poor performance status patients with T790M‑positive advanced non‑small‑cell lung cancer after progression of first- and second‑generation EGFR‑TKI treatments (NEJ032B).Int J Clin Oncol. 2022 Apr;27(4):823-824. doi: 10.1007/s10147-022-02118-8. Int J Clin Oncol. 2022. PMID: 35257278 Free PMC article. No abstract available.
Abstract
Background: Osimertinib is effective in patients with T790M mutation-positive advanced non-small-cell lung cancer (NSCLC) resistant to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). However, its effectiveness and safety in patients with poor performance status (PS) are unknown.
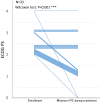
Methods: Enrolled patients showed disease progression after treatment with gefitinib, erlotinib, or afatinib; T790M mutation; stage IIIB, IV, or recurrent disease; and PS of 2-4. Osimertinib was orally administered at a dose of 80 mg/day. The primary endpoint of this phase II study (registration, jRCTs061180018) was response rate and the secondary endpoints were progression-free survival (PFS), overall survival (OS), disease control rate, and safety.
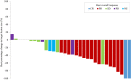
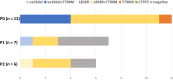
Results: Thirty-three patients were enrolled, of which 69.7% and 24.2% had PS of 2 and 3, respectively. One patient was excluded due to protocol violation; in the remaining 32 patients, the response rate was 53.1%; disease control rate was 75.0%; PFS was 5.1 months; and OS was 10.0 months. The most frequent adverse event of grade 3 or higher severity was lymphopenia (12.1%). Interstitial lung disease (ILD) was observed at all grades and at grades 3-5 in 15.2% (5/33) and 6.1% (2/33) of patients, respectively. Treatment-related death due to ILD occurred in one patient. Patients negative for activating EGFR mutations after osimertinib administration had longer median PFS than those positive for these mutations.
Conclusion: Osimertinib was sufficiently effective in EGFR-TKI-resistant, poor PS patients with T790M mutation-positive advanced NSCLC. Plasma EGFR mutation clearance after TKI treatment could predict the response to EGFR-TKIs.
Keywords: EGFR T790M; Non-small-cell lung cancer; Osimertinib; Phase II; Poor performance status.
© 2021. The Author(s).
Conflict of interest statement
Y.T. received grants and personal fees from Daiichi Sankyo Co. Ltd. and AstraZeneca K.K. and personal fees from Chugai Pharmaceuticals Inc. outside the submitted work. A.N. received personal fees and research funding (institution) from AstraZeneca K.K., Chugai Pharmaceuticals Inc., MSD K.K., and Taiho Pharmaceutical; personal fees from Nippon Boehringer Ingelheim Co. Ltd. and Novartis Pharma K.K.; and research funding (institution) from Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, Takeda Pharmaceutical, and Astellas Pharma Inc. outside the submitted work. H.Y. received personal fees from AstraZeneca K.K., Chugai Pharmaceuticals Inc., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Taiho Pharmaceutical, Ono Pharmaceutical, Delta-Fly Pharma, Inc., Bristol-Myers Squibb Company, Novartis Pharma K.K., Kyowa Kirin Co., Ltd., Nippon Kayaku Co., Ltd., and Eli Lilly Japan K.K. outside the submitted work. S.O. received grants from Abbvie, Amgen, AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Kissei Pharmaceutical, Ono Pharmaceutical, Pfizer, Merck Biopharma, Sanofi, Taiho Pharmaceutical, and Takeda Pharmaceutical, and personal fees from AstraZeneca K.K. and Eli Lilly outside the submitted work. N.F. received personal fees from AstraZeneca K.K., Chugai Pharmaceutical, Nippon Boehringer Ingelheim Co. Ltd., Bristol-Myers Squibb Company, Eli Lilly Japan K.K., MSD K.K., Pfizer Japan Inc. Taiho Pharmaceutical, and Novartis Pharma K.K. outside the submitted work. E.I. received personal fees from AstraZeneca K.K. and Nippon Boehringer Ingelheim Co. Ltd. outside the submitted work. M.M. received personal fees from AstraZeneca K.K., Nippon Boehringer Ingelheim Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Pfizer Japan Inc. outside the submitted work. S.M. received honoraria from AstraZeneca K.K., Bristol-Myers Squibb Company, Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., and Taiho Pharmaceutical Co. Ltd; advisory fees from Astellas Pharma Inc.; and research funding (institution) from Nippon Boehringer Ingelheim Co. Ltd. outside the submitted work. K.K. received personal fees from AstraZeneca K.K., Bristol-Myers Squibb, and Nippon Boehringer Ingelheim Co. Ltd. outside the submitted work. T.I. received grants and personal fees from Daiichi Sankyo Co. Ltd; personal fees from AstraZeneca K.K., Pfizer Japan Inc., and Nippon Boehringer Ingelheim Co., Ltd.; and grants from Pearl Therapeutics Inc. outside the submitted work.
Figures


References
-
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. - DOI - PubMed
-
- Yang JC-H, Wu Y-L, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

