Chloride transport modulators as drug candidates
- PMID: 34644122
- PMCID: PMC8714991
- DOI: 10.1152/ajpcell.00334.2021
Chloride transport modulators as drug candidates
Abstract
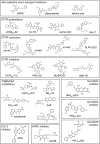
Chloride transport across cell membranes is broadly involved in epithelial fluid transport, cell volume and pH regulation, muscle contraction, membrane excitability, and organellar acidification. The human genome encodes at least 53 chloride-transporting proteins with expression in cell plasma or intracellular membranes, which include chloride channels, exchangers, and cotransporters, some having broad anion specificity. Loss-of-function mutations in chloride transporters cause a wide variety of human diseases, including cystic fibrosis, secretory diarrhea, kidney stones, salt-wasting nephropathy, myotonia, osteopetrosis, hearing loss, and goiter. Although impactful advances have been made in the past decade in drug treatment of cystic fibrosis using small molecule modulators of the defective cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, other chloride channels and solute carrier proteins (SLCs) represent relatively underexplored target classes for drug discovery. New opportunities have emerged for the development of chloride transport modulators as potential therapeutics for secretory diarrheas, constipation, dry eye disorders, kidney stones, polycystic kidney disease, hypertension, and osteoporosis. Approaches to chloride transport-targeted drug discovery are reviewed herein, with focus on chloride channel and exchanger classes in which recent preclinical advances have been made in the identification of small molecule modulators and in proof of concept testing in experimental animal models.
Keywords: chloride channel; chloride exchanger; drug discovery; epithelium; solute carrier proteins.
Conflict of interest statement
Drs. A. S. Verkman and L. J. V. Galietta are named inventors on patent applications for chloride transport modulators, which are owned by their respective universities.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

