The real-world outcomes of multiple myeloma patients treated with daratumumab
- PMID: 34644367
- PMCID: PMC8513840
- DOI: 10.1371/journal.pone.0258487
The real-world outcomes of multiple myeloma patients treated with daratumumab
Abstract
Most patients cannot be included in randomized clinical trials. We report real-world outcomes of all Danish patients with multiple myeloma (MM) treated with daratumumab-based regimens until 1 January 2019.
Methods: Information of 635 patients treated with daratumumab was collected retrospectively and included lines of therapy (LOT), hematologic responses according to the International Myeloma Working Group recommendations, time to next treatment (TNT) and the cause of discontinuation of treatment. Baseline characteristics were acquired from the validated Danish Multiple Myeloma Registry (DMMR).
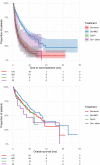
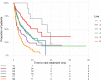
Results: Daratumumab was administrated as monotherapy (Da-mono) in 27.7%, in combination with immunomodulatory drugs (Da-IMiD) in 57.3%, in combination with proteasome inhibitors (Da-PI) in 11.2% and in other combinations (Da-other) in 3.8% of patients. The median number of lines of therapy given before daratumumab was 5 for Da-mono, 3 for Da-IMiD, 4 for Da-PI, and 2 for Da-other. In Da-mono, overall response rate (ORR) was 44.9% and median time to next treatment (mTNT) was 4.9 months. In Da-IMiD, ORR was 80.5%, and mTNT was 16.1 months. In Da-PI, OOR was 60.6% and mTNT was 5.3 months. In patients treated with Da-other, OOR was 54,2% and mTNT was 5.6 months. The use of daratumumab in early LOT was associated with longer TNT (p<0.0001). Patients with amplification 1q had outcome comparable to standard risk patients, while patients with t(4;14), t(14;16) or del17p had worse outcome (p = 0.0001). Multivariate analysis indicated that timing of treatment (timing of daratumumab in the sequence of all LOT that the patients received throughout the course of their disease) was the most important factor for outcome (p<0.0001).
Conclusion: The real-world outcomes of multiple myeloma patients treated with daratumumab are worse than the results of clinical trials. Outcomes achieved with daratumumab were best when daratumumab was used in combination with IMIDs and in early LOT. Patients with high-risk CA had worse outcomes, but patients with amp1q had similar outcomes to standard-risk patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Shah JJ, Abonour R, Gasparetto C, Hardin JW, Toomey K, Narang M, et al. Analysis of Common Eligibility Criteria of Randomized Controlled Trials in Newly Diagnosed Multiple Myeloma Patients and Extrapolating Outcomes. Clinical Lymphoma, Myeloma and Leukemia. 2017;17: 575–583.e2. doi: 10.1016/j.clml.2017.06.013 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

