Late health outcomes after dexrazoxane treatment: A report from the Children's Oncology Group
- PMID: 34644414
- PMCID: PMC8792306
- DOI: 10.1002/cncr.33974
Late health outcomes after dexrazoxane treatment: A report from the Children's Oncology Group
Abstract
Background: The objective of this study was to examine long-term outcomes among children newly diagnosed with cancer who were treated in dexrazoxane-containing clinical trials.
Methods: P9404 (acute lymphoblastic leukemia/lymphoma [ALL]), P9425 and P9426 (Hodgkin lymphoma), P9754 (osteosarcoma), and Dana-Farber Cancer Institute 95-01 (ALL) enrolled 1308 patients between 1996 and 2001: 1066 were randomized (1:1) to doxorubicin with or without dexrazoxane, and 242 (from P9754) were nonrandomly assigned to receive dexrazoxane. Trial data were linked with the National Death Index, the Organ Procurement and Transplantation Network, the Pediatric Health Information System (PHIS), and Medicaid. Osteosarcoma survivors from the Childhood Cancer Survivor Study (CCSS; n = 495; no dexrazoxane) served as comparators in subanalyses. Follow-up events were assessed with cumulative incidence, Cox regression, and Fine-Gray methods.
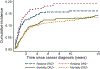
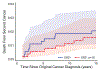
Results: In randomized trials (cumulative prescribed doxorubicin dose, 100-360 mg/m2 ; median follow-up, 18.6 years), dexrazoxane was not associated with relapse (hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.63-1.13), second cancers (HR, 1.19; 95% CI, 0.62-2.30), all-cause mortality (HR, 1.07; 95% CI, 0.78-1.47), or cardiovascular mortality (HR, 1.45; 95% CI, 0.41-5.16). Among P9754 patients (all exposed to dexrazoxane; cumulative doxorubicin, 450-600 mg/m2 ; median follow-up, 16.6-18.4 years), no cardiovascular deaths or heart transplantation occurred. The 20-year heart transplantation rate among CCSS osteosarcoma survivors (mean doxorubicin, 377 ± 145 mg/m2 ) was 1.6% (vs 0% in P9754; P = .13). Among randomized patients, serious cardiovascular outcomes (cardiomyopathy, ischemic heart disease, and stroke) ascertained by PHIS/Medicaid occurred less commonly with dexrazoxane (5.6%) than without it (17.6%; P = .02), although cardiomyopathy rates alone did not differ (4.4% vs 8.1%; P = .35).
Conclusions: Dexrazoxane did not appear to adversely affect long-term mortality, event-free survival, or second cancer risk.
Keywords: adolescent; cancer survivors; cardiotoxicity; child; second malignancy; survivorship.
© 2021 American Cancer Society.
Conflict of interest statement
Figures
References
-
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128: 1927–1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

