Metal-Catalyzed Haloalkynylation Reactions
- PMID: 34644433
- PMCID: PMC9299716
- DOI: 10.1002/chem.202103046
Metal-Catalyzed Haloalkynylation Reactions
Abstract
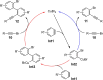
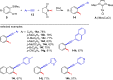
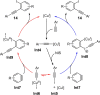
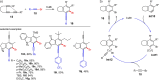
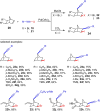
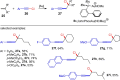
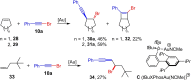
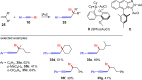
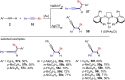
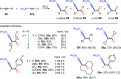
Metal catalysis has revolutionized synthetic chemistry, leading to entirely new, very efficient transformations, which enable access to complex functionalized molecules. One such new transformation method is the haloalkynylation reaction, in which both a halogen atom and an alkynyl unit are transferred to an unsaturated carbon-carbon bond. This minireview summarizes the development of metal-catalyzed haloalkynylation reactions since their beginning about a decade ago. So far, arynes, alkenes and alkynes have been used as unsaturated systems and the reactivities of these systems are summarized in individual chapters of the minireview. Especially, the last few years have witnessed a rapid development due to gold-catalyzed reactions. Here, we discuss how the choice of the catalytic system influences the regio- and stereoselectivity of the addition.
Keywords: C−C coupling; catalysis; haloalkynes; haloalkynylation.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
There is no conflict of interest to declare.
Figures








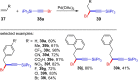
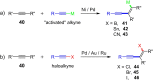
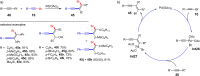




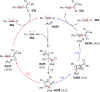
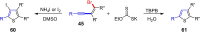
References
-
- Morishita T., Yoshida H., Ohshita J., Chem. Commun. 2010, 46, 640–642. - PubMed
-
- Brand J. P., Waser J., Chem. Soc. Rev. 2012, 41, 4165–4179. - PubMed
-
- Wu W., Jiang H., Acc. Chem. Res. 2014, 47, 2483–2504. - PubMed
-
- H. Jiang, C. Zhu, W. Wu, in Haloalkyne Chemistry, Springer, Heidelberg, 2016.
-
- Petrone D. A., Ye J., Lautens M., Chem. Rev. 2016, 116, 8003–8104. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

