Improved survival of multiple myeloma patients treated with autologous transplantation in the modern era of new medicine
- PMID: 34644446
- PMCID: PMC8645729
- DOI: 10.1111/cas.15163
Improved survival of multiple myeloma patients treated with autologous transplantation in the modern era of new medicine
Abstract
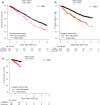
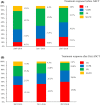
New drugs for multiple myeloma (MM) have dramatically improved patients' overall survival (OS). Autologous stem cell transplantation (ASCT) remains the mainstay for transplant-eligible MM patients. To investigate whether the post-ASCT prognosis of MM patients has been improved by new drugs, we undertook a retrospective observational analysis using the Transplant Registry Unified Management Program database in Japan. We analyzed 7323 patients (4135 men and 3188 women; median age, 59 years; range 16-77 years) who underwent upfront ASCT between January 2007 and December 2018. We categorized them by when they underwent ASCT according to the drugs' introduction in Japan: group 1 (2007-2010), group 2 (2011-2016), and group 3 (2017-2018). We compared the groups' post-ASCT OS. The 2-year OS rates (95% confidence interval [CI]) of groups 1, 2, and 3 were 85.8% (84.1%-87.4%), 89.1% (88.0%-90.1%), and 92.3% (90.0%-94.2%) (P < .0001) and the 5-year OS (95% CI) rates were 64.9% (62.4%-67.3%), 71.6% (69.7%-73.3%), and not applicable, respectively (P < .0001). A multivariate analysis showed that the post-ASCT OS was superior with these factors: age less than 65 years, performance status 0/1, low International Staging System (ISS) stage, receiving SCT for 180 days or less post-diagnosis, better treatment response pre-ASCT, later year of ASCT, and receiving SCT twice. A subgroup analysis showed poor prognoses for the patients with unfavorable karyotype and poor treatment response post-ASCT. The post-ASCT OS has thus improved over time (group 1 < 2 < 3) with the introduction of new drugs for MM. As the prognosis of high-risk-karyotype patients with ISS stage III remains poor, their treatment requires improvement.
Keywords: autologous stem cell transplantation; multiple myeloma; new medicine; overall survival; prognosis.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



References
-
- Japanese Society of Hematology . Practical Guidelines for Hematological Malignancies, 2018, Revised Version, 2nd edn. Kanehara Shuppan; 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

