TRIP12 ubiquitination of glucocerebrosidase contributes to neurodegeneration in Parkinson's disease
- PMID: 34644545
- PMCID: PMC8639668
- DOI: 10.1016/j.neuron.2021.09.031
TRIP12 ubiquitination of glucocerebrosidase contributes to neurodegeneration in Parkinson's disease
Abstract
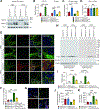
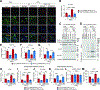
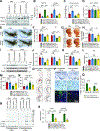
Impairment in glucocerebrosidase (GCase) is strongly associated with the development of Parkinson's disease (PD), yet the regulators responsible for its impairment remain elusive. In this paper, we identify the E3 ligase Thyroid Hormone Receptor Interacting Protein 12 (TRIP12) as a key regulator of GCase. TRIP12 interacts with and ubiquitinates GCase at lysine 293 to control its degradation via ubiquitin proteasomal degradation. Ubiquitinated GCase by TRIP12 leads to its functional impairment through premature degradation and subsequent accumulation of α-synuclein. TRIP12 overexpression causes mitochondrial dysfunction, which is ameliorated by GCase overexpression. Further, conditional TRIP12 knockout in vitro and knockdown in vivo promotes the expression of GCase, which blocks α-synuclein preformed fibrils (α-syn PFFs)-provoked dopaminergic neurodegeneration. Moreover, TRIP12 accumulates in human PD brain and α-synuclein-based mouse models. The identification of TRIP12 as a regulator of GCase provides a new perspective on the molecular mechanisms underlying dysfunctional GCase-driven neurodegeneration in PD.
Keywords: Gaucher’s disease (GD); Parkinson’s disease (PD); Thyroid Hormone Receptor Interacting Protein 12 (TRIP12); glucocerebrosidase (GCase); glucocerebrosidase 1 gene (GBA1); glucosylceramide (GlcCer); lysosome; mitochondria; α-synuclein; α-synuclein preformed fibrils (α-syn PFFs).
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Bramswig NC, Ludecke HJ, Pettersson M, Albrecht B, Bernier RA, Cremer K, Eichler EE, Falkenstein D, Gerdts J, Jansen S, et al. (2017). Identification of new TRIP12 variants and detailed clinical evaluation of individuals with non-syndromic intellectual disability with or without autism. Hum Genet 136, 179–192. 10.1007/s00439-016-1743-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

