Potentiated lung adenocarcinoma (LUAD) cell growth, migration and invasion by lncRNA DARS-AS1 via miR-188-5p/ KLF12 axis
- PMID: 34644678
- PMCID: PMC8544313
- DOI: 10.18632/aging.203632
Potentiated lung adenocarcinoma (LUAD) cell growth, migration and invasion by lncRNA DARS-AS1 via miR-188-5p/ KLF12 axis
Abstract
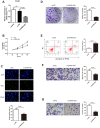
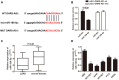
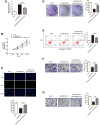
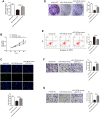
Lung adenocarcinoma (LUAD) is the most common histological type of non-small cell lung cancer (NSCLC). Due to the nonspecific early symptoms, the majority of the diagnosed LUAD patients are in the middle and late stages, with multiple metastases, and have missed the optimal period for treatment. Current studies have reported lncRNA DARS-AS1 as a cancer-promoting gene that expedites tumorigenesis. This is the first study demonstrating that DARS-AS1 is involved in the mediating process of LUAD. Cell functional experiments revealed that lncRNA DARS-AS1 participated in enhancing LUAD proliferation, invasion, and migration by inhibiting miR-188-5p. The investigation on DARS-AS1/miR-188-5p led to the discovery of KLF12 as a downstream target of miR-188-5p, and the regulatory pathway was established as DARS-AS1/miR-188-5p/KLF12. According to western blot results, DARS-AS1 promoted LUAD cell growth, migration, and invasion via stimulation of the PI3K/AKT pathway, activating the EMT process, and up-regulating the CyclinD1 and Bcl-2 proteins. This was the first report on the DARS-AS1/miR-188-5p/KLF12 axis and offered a novel strategy for early diagnosis, a new therapeutic method, and an improved prognosis for LUAD.
Keywords: DARS-AS1; Krüppel-like factor 12; lncRNA; lung adenocarcinoma; miR-188-5p.
Conflict of interest statement
Figures





Similar articles
-
LncRNA SGMS1-AS1 regulates lung adenocarcinoma cell proliferation, migration, invasion, and EMT progression via miR-106a-5p/MYLI9 axis.Thorac Cancer. 2021 Jul;12(14):2104-2112. doi: 10.1111/1759-7714.14043. Epub 2021 Jun 1. Thorac Cancer. 2021. PMID: 34061466 Free PMC article.
-
FAM83A-AS1 promotes lung adenocarcinoma cell migration and invasion by targeting miR-150-5p and modifying MMP14.Cell Cycle. 2019 Nov;18(21):2972-2985. doi: 10.1080/15384101.2019.1664225. Epub 2019 Sep 16. Cell Cycle. 2019. PMID: 31522616 Free PMC article.
-
LncRNA TTN-AS1 promotes migration, invasion, and epithelial mesenchymal transition of lung adenocarcinoma via sponging miR-142-5p to regulate CDK5.Cell Death Dis. 2019 Jul 30;10(8):573. doi: 10.1038/s41419-019-1811-y. Cell Death Dis. 2019. PMID: 31363080 Free PMC article.
-
LncRNA LOXL1-AS1 regulates the tumorigenesis and development of lung adenocarcinoma through sponging miR-423-5p and targeting MYBL2.Cancer Med. 2020 Jan;9(2):689-699. doi: 10.1002/cam4.2641. Epub 2019 Nov 23. Cancer Med. 2020. PMID: 31758653 Free PMC article.
-
DARS-AS1: A Vital Oncogenic LncRNA Regulator with Potential for Cancer Prognosis and Therapy.Int J Med Sci. 2024 Jan 20;21(3):571-582. doi: 10.7150/ijms.90611. eCollection 2024. Int J Med Sci. 2024. PMID: 38322590 Free PMC article. Review.
Cited by
-
Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD).Biomedicines. 2023 Jun 29;11(7):1861. doi: 10.3390/biomedicines11071861. Biomedicines. 2023. PMID: 37509501 Free PMC article.
-
Identification of molecular subtypes and a prognostic signature based on m6A/m5C/m1A-related genes in lung adenocarcinoma.Sci Rep. 2024 Mar 30;14(1):7543. doi: 10.1038/s41598-024-57910-5. Sci Rep. 2024. PMID: 38555384 Free PMC article.
-
SLC7A8 overexpression inhibits the growth and metastasis of lung adenocarcinoma and is correlated with a dismal prognosis.Aging (Albany NY). 2024 Jan 18;16(2):1605-1619. doi: 10.18632/aging.205446. Epub 2024 Jan 18. Aging (Albany NY). 2024. PMID: 38244585 Free PMC article.
-
A novel prognostic signature for lung adenocarcinoma based on cuproptosis-related lncRNAs: A Review.Medicine (Baltimore). 2022 Dec 9;101(49):e31924. doi: 10.1097/MD.0000000000031924. Medicine (Baltimore). 2022. PMID: 36626411 Free PMC article. Review.
-
Sodium tanshinone IIA sulphate inhibits angiogenesis in lung adenocarcinoma via mediation of miR-874/eEF-2K/TG2 axis.Pharm Biol. 2023 Dec;61(1):868-877. doi: 10.1080/13880209.2023.2204879. Pharm Biol. 2023. PMID: 37300283 Free PMC article.
References
-
- Ariozzi I, Paladini I, Gnetti L, Silva M, Colombi D, De Filippo M, Sverzellati N. Computed tomography-histologic correlations in lung cancer. Pathologica. 2013; 105:329–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

