Motor function in type 2 and 3 SMA patients treated with Nusinersen: a critical review and meta-analysis
- PMID: 34645478
- PMCID: PMC8515709
- DOI: 10.1186/s13023-021-02065-z
Motor function in type 2 and 3 SMA patients treated with Nusinersen: a critical review and meta-analysis
Abstract
Background: There is an increasing number of papers reporting the real world use of Nusinersen in different cohorts of SMA patients.
Main body: The aim of this paper was to critically review the literature reporting real world data on motor function in type 2 and 3 patients treated with Nusinersen, subdividing the results according to SMA type, age and type of assessment and performing a meta-analysis of the available results. We also report the available data collected in untreated patients using the same measures. Of the 400 papers identified searching for Nusinersen and spinal muscular atrophy, 19 reported motor function in types 2 and 3: 13 in adults, 4 in children and 2 included both. Twelve papers reported untreated patients' data. All studies reported positive changes on at least one of the functional measures and at every time point while all-untreated cohorts showed negative changes.
Conclusion: Our review suggests that Nusinersen provides a favorable benefit in motor function across a wide range of SMA type 2 and 3 patients over a 10-14 month observation period. Although a direct comparison with studies reporting data from untreated patients cannot be made, the longitudinal changes in the treated cohorts (consistently positive) are divergent from those observed in the untreated cohorts (consistently negative). The difference could be observed both in the global cohorts and in smaller groups subdivided according to age, type or functional status.
Keywords: Critical review; Nusinersen; Spinal muscular atrophy.
© 2021. The Author(s).
Conflict of interest statement
Authors GC, MCP, FB, VS, RF, MPa, EM, reports personal fees BIOGEN S.R.L., ROCHE, AVEXIS, NOVARTIS outside the submitted work; Authors CC, MPo, FC, LA have nothing to disclose.
Figures
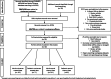


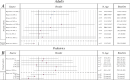
References
-
- Day JW, Finkel RS, Chiriboga CA, Connolly AM, Crawford TO, Darras BT, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi: 10.1016/S1474-4422(21)00001-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

