Conduct and reporting of formula milk trials: systematic review
- PMID: 34645600
- PMCID: PMC8513520
- DOI: 10.1136/bmj.n2202
Conduct and reporting of formula milk trials: systematic review
Erratum in
-
Conduct and reporting of formula milk trials: systematic review.BMJ. 2021 Nov 24;375:n2890. doi: 10.1136/bmj.n2890. BMJ. 2021. PMID: 34819301 Free PMC article. No abstract available.
Abstract
Objective: To systematically review the conduct and reporting of formula trials.
Design: Systematic review.
Data sources: Medline, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from 1 January 2006 to 31 December 2020.
Review methods: Intervention trials comparing at least two formula products in children less than three years of age were included, but not trials of human breast milk or fortifiers of breast milk. Data were extracted in duplicate and primary outcome data were synthesised for meta-analysis with a random effects model weighted by the inverse variance method. Risk of bias was evaluated with Cochrane risk of bias version 2.0, and risk of undermining breastfeeding was evaluated according to published consensus guidance. Primary outcomes of the trials included in the systematic review were identified from clinical trial registries, protocols, or trial publications.
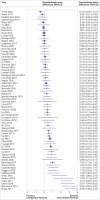
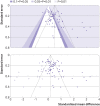
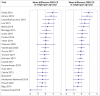
Results: 22 201 titles were screened and 307 trials were identified that were published between 2006 and 2020, of which 73 (24%) trials in 13 197 children were prospectively registered. Another 111 unpublished but registered trials in 17 411 children were identified. Detailed analysis was undertaken for 125 trials (23 757 children) published since 2015. Seventeen (14%) of these recently published trials were conducted independently of formula companies, 26 (21%) were prospectively registered with a clear aim and primary outcome, and authors or sponsors shared prospective protocols for 11 (9%) trials. Risk of bias was low in five (4%) and high in 100 (80%) recently published trials, mainly because of inappropriate exclusions from analysis and selective reporting. For 68 recently published superiority trials, a pooled standardised mean difference of 0.51 (range -0.43 to 3.29) was calculated with an asymmetrical funnel plot (Egger's test P<0.001), which reduced to 0.19 after correction for asymmetry. Primary outcomes were reported by authors as favourable in 86 (69%) trials, and 115 (92%) abstract conclusions were favourable. One of 38 (3%) trials in partially breastfed infants reported adequate support for breastfeeding and 14 of 87 (16%) trials in non-breastfed infants confirmed the decision not to breastfeed was firmly established before enrolment in the trial.
Conclusions: The results show that formula trials lack independence or transparency, and published outcomes are biased by selective reporting.
Systematic review registration: PROSPERO 2018 CRD42018091928.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare support from Imperial Health Charity: RJB received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. JL-B received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work. The University of Colorado receives remuneration for LB’s work as senior editor, Cochrane, which is outside the submitted work. The authors report no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Baker P, Santos T, Neves PA, et al. First-food systems transformations and the ultra-processing of infant and young child diets: The determinants, dynamics and consequences of the global rise in commercial milk formula consumption. Matern Child Nutr 2021;17:e13097. 10.1111/mcn.13097. - DOI - PMC - PubMed
-
- Tran HT, Nguyen TT, Mathisen R. The use of human donor milk. BMJ 2020;371:m4243. 10.1136/bmj.m4243. - DOI
-
- Infant formula requirements pertaining to current good manufacturing practice, quality control procedures, quality factors, records and reports, and notifications. Electronic Code of Federal Regulations, Title 21, Chapter IB, Part 106.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
