Broadband Dynamics Rather than Frequency-Specific Rhythms Underlie Prediction Error in the Primate Auditory Cortex
- PMID: 34645605
- PMCID: PMC8580146
- DOI: 10.1523/JNEUROSCI.0367-21.2021
Broadband Dynamics Rather than Frequency-Specific Rhythms Underlie Prediction Error in the Primate Auditory Cortex
Abstract
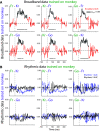
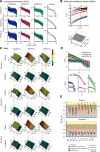
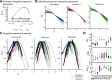
Detection of statistical irregularities, measured as a prediction error response, is fundamental to the perceptual monitoring of the environment. We studied whether prediction error response is associated with neural oscillations or asynchronous broadband activity. Electrocorticography was conducted in three male monkeys, who passively listened to the auditory roving oddball stimuli. Local field potentials (LFPs) recorded over the auditory cortex underwent spectral principal component analysis, which decoupled broadband and rhythmic components of the LFP signal. We found that the broadband component captured the prediction error response, whereas none of the rhythmic components were associated with statistical irregularities of sounds. The broadband component displayed more stochastic, asymmetrical multifractal properties than the rhythmic components, which revealed more self-similar dynamics. We thus conclude that the prediction error response is captured by neuronal populations generating asynchronous broadband activity, defined by irregular dynamic states, which, unlike oscillatory rhythms, appear to enable the neural representation of auditory prediction error response.SIGNIFICANCE STATEMENT This study aimed to examine the contribution of oscillatory and asynchronous components of auditory local field potentials in the generation of prediction error responses to sensory irregularities, as this has not been directly addressed in the previous studies. Here, we show that mismatch negativity-an auditory prediction error response-is driven by the asynchronous broadband component of potentials recorded in the auditory cortex. This finding highlights the importance of nonoscillatory neural processes in the predictive monitoring of the environment. At a more general level, the study demonstrates that stochastic neural processes, which are often disregarded as neural noise, do have a functional role in the processing of sensory information.
Keywords: auditory cortex; broadband response; mismatch negativity; multiscale multifractal analysis; prediction error; rhythmic components.
Copyright © 2021 the authors.
Figures



References
-
- Alho K (1995) Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear 16:38–51. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
