Clinical validation of automated and rapid mariPOC SARS-CoV-2 antigen test
- PMID: 34645929
- PMCID: PMC8514458
- DOI: 10.1038/s41598-021-99886-6
Clinical validation of automated and rapid mariPOC SARS-CoV-2 antigen test
Abstract
COVID-19 diagnostics was quickly ramped up worldwide early 2020 based on the detection of viral RNA. However, based on the scientific knowledge for pre-existing coronaviruses, it was expected that the SARS-CoV-2 RNA will be detected from symptomatic and at significant rates also from asymptomatic individuals due to persistence of non-infectious RNA. To increase the efficacy of diagnostics, surveillance, screening and pandemic control, rapid methods, such as antigen tests, are needed for decentralized testing and to assess infectiousness. A novel automated mariPOC SARS-CoV-2 test was developed for the detection of conserved structural viral nucleocapsid proteins. The test utilizes sophisticated optical laser technology for two-photon excitation and individual detection of immunoassay solid-phase particles. We validated the new method against qRT-PCR. Sensitivity of the test was 100.0% (13/13) directly from nasopharyngeal swab specimens and 84.4% (38/45) from swab specimens in undefined transport mediums. Specificity of the test was 100.0% (201/201). The test's limit of detection was 2.7 TCID50/test. It showed no cross-reactions. Our study shows that the new test can detect infectious individuals already in 20 min with clinical sensitivity close to qRT-PCR. The mariPOC is a versatile platform for syndromic testing and for high capacity infection control screening of infectious individuals.
© 2021. The Author(s).
Conflict of interest statement
JMK, PA and JOK are employees at ArcDia International Ltd. KH, AH, NI, CS-K and KS declare no competing interests.
Figures
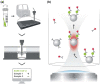
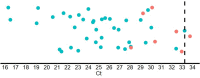
References
-
- Gaunt ER, Hardie A, Claas ECJ, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J. Clin. Microbiol. 2010;48:2940–2947. doi: 10.1128/jcm.00636-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

