Oestrogen engages brain MC4R signalling to drive physical activity in female mice
- PMID: 34646010
- PMCID: PMC9113400
- DOI: 10.1038/s41586-021-04010-3
Oestrogen engages brain MC4R signalling to drive physical activity in female mice
Abstract
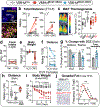
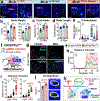
Oestrogen depletion in rodents and humans leads to inactivity, fat accumulation and diabetes1,2, underscoring the conserved metabolic benefits of oestrogen that inevitably decrease with age. In rodents, the preovulatory surge in 17β-oestradiol (E2) temporarily increases energy expenditure to coordinate increased physical activity with peak sexual receptivity. Here we report that a subset of oestrogen-sensitive neurons in the ventrolateral ventromedial hypothalamic nucleus (VMHvl)3-7 projects to arousal centres in the hippocampus and hindbrain, and enables oestrogen to rebalance energy allocation in female mice. Surges in E2 increase melanocortin-4 receptor (MC4R) signalling in these VMHvl neurons by directly recruiting oestrogen receptor-α (ERα) to the Mc4r gene. Sedentary behaviour and obesity in oestrogen-depleted female mice were reversed after chemogenetic stimulation of VMHvl neurons expressing both MC4R and ERα. Similarly, a long-term increase in physical activity is observed after CRISPR-mediated activation of this node. These data extend the effect of MC4R signalling - the most common cause of monogenic human obesity8 - beyond the regulation of food intake and rationalize reported sex differences in melanocortin signalling, including greater disease severity of MC4R insufficiency in women9. This hormone-dependent node illuminates the power of oestrogen during the reproductive cycle in motivating behaviour and maintaining an active lifestyle in women.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures


Comment in
-
A two-step hormone-signalling mechanism that drives physical activity.Nature. 2021 Nov;599(7883):37-39. doi: 10.1038/d41586-021-02725-x. Nature. 2021. PMID: 34646025 No abstract available.
-
Estrogen wars: The activity awakens.Cell Metab. 2021 Dec 7;33(12):2309-2311. doi: 10.1016/j.cmet.2021.11.006. Cell Metab. 2021. PMID: 34879237
References
-
- Carr MC The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88, 2404–2411 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK121657/DK/NIDDK NIH HHS/United States
- R01 MH109907/MH/NIMH NIH HHS/United States
- 2T32 GM065094/NH/NIH HHS/United States
- K01 DK098320/DK/NIDDK NIH HHS/United States
- K12 GM081266/GM/NIGMS NIH HHS/United States
- T32 GM065094/GM/NIGMS NIH HHS/United States
- P30 DK098722/DK/NIDDK NIH HHS/United States
- F31 MH124365/MH/NIMH NIH HHS/United States
- R01 MH113628/MH/NIMH NIH HHS/United States
- AHA/American Heart Association-American Stroke Association/United States
- R01 AG062331/AG/NIA NIH HHS/United States
- R01 CA197139/CA/NCI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- T32 NS115706/NS/NINDS NIH HHS/United States
- R01 EY030138/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

