Unravelling the collateral damage of antibiotics on gut bacteria
- PMID: 34646011
- PMCID: PMC7612847
- DOI: 10.1038/s41586-021-03986-2
Unravelling the collateral damage of antibiotics on gut bacteria
Abstract
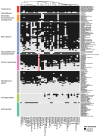
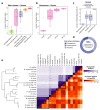
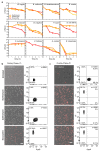
Antibiotics are used to fight pathogens but also target commensal bacteria, disturbing the composition of gut microbiota and causing dysbiosis and disease1. Despite this well-known collateral damage, the activity spectrum of different antibiotic classes on gut bacteria remains poorly characterized. Here we characterize further 144 antibiotics from a previous screen of more than 1,000 drugs on 38 representative human gut microbiome species2. Antibiotic classes exhibited distinct inhibition spectra, including generation dependence for quinolones and phylogeny independence for β-lactams. Macrolides and tetracyclines, both prototypic bacteriostatic protein synthesis inhibitors, inhibited nearly all commensals tested but also killed several species. Killed bacteria were more readily eliminated from in vitro communities than those inhibited. This species-specific killing activity challenges the long-standing distinction between bactericidal and bacteriostatic antibiotic classes and provides a possible explanation for the strong effect of macrolides on animal3-5 and human6,7 gut microbiomes. To mitigate this collateral damage of macrolides and tetracyclines, we screened for drugs that specifically antagonized the antibiotic activity against abundant Bacteroides species but not against relevant pathogens. Such antidotes selectively protected Bacteroides species from erythromycin treatment in human-stool-derived communities and gnotobiotic mice. These findings illluminate the activity spectra of antibiotics in commensal bacteria and suggest strategies to circumvent their adverse effects on the gut microbiota.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
EMBL has filed a patent on using the antidotes identified in this study for prevention and/or treatment of dysbiosis and for microbiome protection (European patent application number EP19216548.8). LM, CVG, EC and AT are listed as inventors.
Figures











References
(Method part)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

