Neural control of affiliative touch in prosocial interaction
- PMID: 34646019
- PMCID: PMC8605624
- DOI: 10.1038/s41586-021-03962-w
Neural control of affiliative touch in prosocial interaction
Abstract
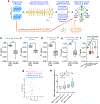
The ability to help and care for others fosters social cohesiveness and is vital to the physical and emotional well-being of social species, including humans1-3. Affiliative social touch, such as allogrooming (grooming behaviour directed towards another individual), is a major type of prosocial behaviour that provides comfort to others1-6. Affiliative touch serves to establish and strengthen social bonds between animals and can help to console distressed conspecifics. However, the neural circuits that promote prosocial affiliative touch have remained unclear. Here we show that mice exhibit affiliative allogrooming behaviour towards distressed partners, providing a consoling effect. The increase in allogrooming occurs in response to different types of stressors and can be elicited by olfactory cues from distressed individuals. Using microendoscopic calcium imaging, we find that neural activity in the medial amygdala (MeA) responds differentially to naive and distressed conspecifics and encodes allogrooming behaviour. Through intersectional functional manipulations, we establish a direct causal role of the MeA in controlling affiliative allogrooming and identify a select, tachykinin-expressing subpopulation of MeA GABAergic (γ-aminobutyric-acid-expressing) neurons that promote this behaviour through their projections to the medial preoptic area. Together, our study demonstrates that mice display prosocial comforting behaviour and reveals a neural circuit mechanism that underlies the encoding and control of affiliative touch during prosocial interactions.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures



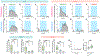



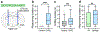





Comment in
-
Comforting in mice.Nat Neurosci. 2021 Dec;24(12):1640. doi: 10.1038/s41593-021-00979-2. Nat Neurosci. 2021. PMID: 34848880 No abstract available.
References
-
- de Waal FBM & Preston SD Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18, 498–509 (2017). - PubMed
-
- Rault J-L Be kind to others: Prosocial behaviours and their implications for animal welfare. Appl Anim Behav Sci 210, 113–123 (2019).
-
- Morrison I Keep Calm and Cuddle on: Social Touch as a Stress Buffer. Adapt Hum Behav Physiology 2, 344–362 (2016).
-
- Spruijt BM, Hooff J. A. van & Gispen WH Ethology and neurobiology of grooming behavior. Physiol Rev 72, 825–852 (1992). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

