Lessons in self-defence: inhibition of virus entry by intrinsic immunity
- PMID: 34646033
- PMCID: PMC8511856
- DOI: 10.1038/s41577-021-00626-8
Lessons in self-defence: inhibition of virus entry by intrinsic immunity
Abstract
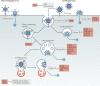
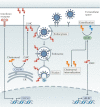
Virus entry, consisting of attachment to and penetration into the host target cell, is the first step of the virus life cycle and is a critical 'do or die' event that governs virus emergence in host populations. Most antiviral vaccines induce neutralizing antibodies that prevent virus entry into cells. However, while the prevention of virus invasion by humoral immunity is well appreciated, considerably less is known about the immune defences present within cells (known as intrinsic immunity) that interfere with virus entry. The interferon-induced transmembrane (IFITM) proteins, known for inhibiting fusion between viral and cellular membranes, were once the only factors known to restrict virus entry. However, the progressive development of genetic and pharmacological screening platforms and the onset of the COVID-19 pandemic have galvanized interest in how viruses infiltrate cells and how cells defend against it. Several host factors with antiviral potential are now implicated in the regulation of virus entry, including cholesterol 25-hydroxylase (CH25H), lymphocyte antigen 6E (LY6E), nuclear receptor co-activator protein 7 (NCOA7), interferon-γ-inducible lysosomal thiol reductase (GILT), CD74 and ARFGAP with dual pleckstrin homology domain-containing protein 2 (ADAP2). This Review summarizes what is known and what remains to be understood about the intrinsic factors that form the first line of defence against virus infection.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

