Acute Hepatic Porphyria: Pathophysiological Basis of Neuromuscular Manifestations
- PMID: 34646118
- PMCID: PMC8502968
- DOI: 10.3389/fnins.2021.715523
Acute Hepatic Porphyria: Pathophysiological Basis of Neuromuscular Manifestations
Abstract
Acute hepatic porphyria represents a rare, underdiagnosed group of inherited metabolic disorders due to hereditary defects of heme group biosynthesis pathway. Most patients have their definite diagnosis after several years of complex and disabling clinical manifestations and commonly after life-threatening acute neurovisceral episodes or severe motor handicap. Many key studies in the last two decades have been performed and led to the discovery of novel possible diagnostic and prognostic biomarkers and to the development of new therapeutic purposes, including small interfering RNA-based therapy, specifically driven to inhibit selectively delta-aminolevulinic acid synthase production and decrease the recurrence number of severe acute presentation for most patients. Several distinct mechanisms have been identified to contribute to the several neuromuscular signs and symptoms. This review article aims to present the current knowledge regarding the main pathophysiological mechanisms involved with the acute and chronic presentation of acute hepatic porphyria and to highlight the relevance of such content for clinical practice and in decision making about therapeutic options.
Keywords: acute hepatic porphyria; dysautonomia; inborn errors of metabolism (IEM); inherited metabolic diseases; neuromuscular; neuropathy; pathophysiology; rhabdomyolysis.
Copyright © 2021 de Souza, Badia, Farias, Pinto and Oliveira.
Conflict of interest statement
PS had received honorarium from Alnylam Pharmaceuticals as speaker and scientific advisory boarding member. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

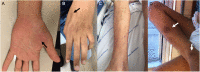

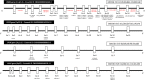
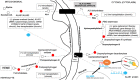
References
-
- Adams C. D., Amaya P. F. (2014). Quadriparesis and rhabdomyolysis due to acute intermittent porphyria: case report. Acta Med. Colomb. 39:28.
Publication types
LinkOut - more resources
Full Text Sources

