Lactobacillus casei relieves liver injury by regulating immunity and suppression of the enterogenic endotoxin-induced inflammatory response in rats cotreated with alcohol and iron
- PMID: 34646510
- PMCID: PMC8497841
- DOI: 10.1002/fsn3.2486
Lactobacillus casei relieves liver injury by regulating immunity and suppression of the enterogenic endotoxin-induced inflammatory response in rats cotreated with alcohol and iron
Abstract
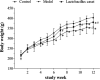
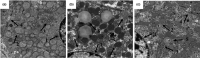
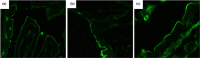
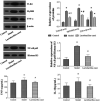
Excessive alcohol and iron intake can reportedly cause liver damage. In the present study, we investigated the effect of Lactobacillus casei on liver injury in rats co-exposed to alcohol and iron and evaluated its possible mechanism. Sixty male Wistar rats were randomly divided into three groups for 12 weeks: the Control group (administered normal saline by gavage and provided a normal diet); alcohol +iron group (Model group, treated with alcohol [3.5-5.3 g/kg/day] by gavage and dietary iron [1,500 mg/kg]); Model group supplemented with L. casei (8 × 108 CFU kg-1 day-1) (L. casei group). Using hematoxylin and eosin (HE) staining and transmission electron microscopy, we observed that L. casei supplementation could alleviate disorders associated with lipid metabolism, inflammation, and intestinal mucosal barrier injury. Moreover, levels of serum alanine aminotransferase, gamma-glutamyl transferase, triglyceride (TG), and hepatic TG were significantly increased in the model group; however, these levels were significantly decreased following the 12-week L. casei supplementation. In addition, we observed notable improvements in intestinal mucosal barrier function and alterations in T lymphocyte subsets and natural killer cells in L. casei-treated rats when compared with the model group. Furthermore, L. casei intervention alleviated serum levels of tumor necrosis factor-α and interleukin-1β, accompanied by decreased serum endotoxin levels and downregulated expression of toll-like receptor 4 and its related molecules MyD88, nuclear factor kappa-B p65, and TNF-α. Accordingly, supplementation with L. casei could effectively improve liver injury induced by the synergistic interaction between alcohol and iron. The underlying mechanism for this improvement may be related to immune regulation and inhibition of enterogenic endotoxin-mediated inflammation.
Keywords: Lactobacillus casei; alcohol and iron; immunity; inflammatory; liver injury.
© 2021 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Effects of Lactobacillus casei on Iron Metabolism and Intestinal Microflora in Rats Exposed to Alcohol and Iron.Turk J Gastroenterol. 2022 Jun;33(6):470-476. doi: 10.5152/tjg.2022.21370. Turk J Gastroenterol. 2022. PMID: 35786614 Free PMC article.
-
Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure.Eur J Nutr. 2016 Mar;55(2):821-831. doi: 10.1007/s00394-015-0904-3. Epub 2015 Apr 18. Eur J Nutr. 2016. PMID: 25893720
-
Dietary Supplementation with Lactobacillus casei Alleviates Lipopolysaccharide-Induced Liver Injury in a Porcine Model.Int J Mol Sci. 2017 Nov 26;18(12):2535. doi: 10.3390/ijms18122535. Int J Mol Sci. 2017. PMID: 29186870 Free PMC article.
-
Regulation of Gut Microflora by Lactobacillus casei Zhang Attenuates Liver Injury in Mice Caused by Anti-Tuberculosis Drugs.Int J Mol Sci. 2023 May 29;24(11):9444. doi: 10.3390/ijms24119444. Int J Mol Sci. 2023. PMID: 37298396 Free PMC article.
-
Antiobesity Effects of Lactobacillus paracasei Subsp. paracasei, L. casei 431 on High-Fat Diet-Induced Obese Rats.J Med Food. 2023 Jul;26(7):445-453. doi: 10.1089/jmf.2022.K.0144. Epub 2023 Jun 13. J Med Food. 2023. PMID: 37311176
Cited by
-
Microbial treatment of alcoholic liver disease: A systematic review and meta-analysis.Front Nutr. 2022 Nov 21;9:1054265. doi: 10.3389/fnut.2022.1054265. eCollection 2022. Front Nutr. 2022. PMID: 36479298 Free PMC article.
-
Lactobacillus reuteri FN041 Alleviates Radiation Enteritis by Regulating M2 Macrophages Polarization Based on Gut Microbiota and Its Metabolites.Food Sci Nutr. 2025 Aug 27;13(9):e70871. doi: 10.1002/fsn3.70871. eCollection 2025 Sep. Food Sci Nutr. 2025. PMID: 40895137 Free PMC article.
-
Suppression of P2X4 and P2X7 by Lactobacillus rhamnosus vitaP1: effects on hangover symptoms.AMB Express. 2024 Mar 15;14(1):30. doi: 10.1186/s13568-024-01685-5. AMB Express. 2024. PMID: 38491208 Free PMC article.
-
The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice.Sci Rep. 2023 Aug 16;13(1):13278. doi: 10.1038/s41598-023-39279-z. Sci Rep. 2023. PMID: 37587146 Free PMC article.
-
Effects of Moderate Consumption of a Probiotic-Fermented Sour Beer on the Inflammatory, Immunity, Lipid Profile, and Gut Microbiome of Healthy Men in a Participant-Blinded, Randomized-Controlled Within-Subject Crossover Study.Food Sci Nutr. 2024 Dec 1;12(12):10867-10880. doi: 10.1002/fsn3.4627. eCollection 2024 Dec. Food Sci Nutr. 2024. PMID: 39723031 Free PMC article.
References
-
- Buracco, S. , Peracino, B. , Andreini, C. , Bracco, E. , & Bozzaro, S. (2017). Differential effects of iron, zinc, and copper on Dictyostelium discoideum cell growth and resistance to Legionella pneumophila. Frontiers in Cellular and Infection Microbiology, 7, 536. 10.3389/fcimb.2017.00536 - DOI - PMC - PubMed
-
- Chen, J. , Lu, W.‐Y. , Zhao, M.‐F. , Cao, X.‐L. , Jiang, Y.‐Y. , Jin, X. , Xu, P. , Yuan, T.‐T. , Zhang, Y.‐C. , Chai, X. , Meng, J.‐X. , Li, Q. , Xiao, X. , Mu, J. , Li, D.‐G. , & Qi, A.‐P. (2017). Reactive oxygen species mediated T lymphocyte abnormalities in an iron‐overloaded mouse model and iron‐overloaded patients with myelodysplastic syndromes. Annals of Hematology, 96(7), 1085–1095. 10.1007/s00277-017-2985-y - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

