Chitotriosidase as a biomarker for gangliosidoses
- PMID: 34646735
- PMCID: PMC8498089
- DOI: 10.1016/j.ymgmr.2021.100803
Chitotriosidase as a biomarker for gangliosidoses
Abstract
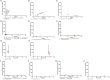
Elevated serum chitotriosidase (CHITO) is an indication of macrophage activation, and its capacity have been explored as a marker of inflammation in a number of disease states. For over a decade, CHITO plasma levels have been used by clinicians as a biomarker of inflammation in the lysosomal disease, Gaucher disease, including monitoring response to therapies in patients with Gaucher disease type I. Although it is becoming increasingly recognized that inflammation is a prominent component of many lysosomal diseases, the relation of CHITO levels to disease burden has not been well-characterized in the large majority of lysosomal diseases. Moreover, the role of CHITO in lysosomal diseases that affect the central nervous system (CNS) has not been systematically studied. In this study, one hundred and thirty-four specimens of CSF and serum were collected from 34 patients with lysosomal diseases affecting the CNS. This study included patients with GM1-gangliosidosis, GM2-gangliosidosis, mucopolysaccharidoses (MPS), multiple sulfatase deficiency and Gaucher disease. CHITO levels in the CSF were significantly higher in patients with more rapidly progressing severe neurological impairment: GM1-gangliosidosis vs MPS (p < 0.0001); GM2-gangliosidosis vs MPS (p < 0.0001). CHITO levels were higher in patients with the more severe phenotypes compared to milder phenotypes in GM1-gangliosidosis and GM2-gangliosidosis (serum CHITO in GM1-gangliosidosis infantile vs juvenile p = 0.025; CSF CHITO in Tay-Sachs infantile vs Tay-Sachs late-onset p < 0.0001). Moreover, higher CHITO levels in the CSF were significantly associated with lower cognitive test scores in patients with GM1-gangliosidosis, GM2-gangliosidosis, and MPS (p = 1.12*10-5, R2 = 0.72). Patients with infantile GM1-gangliosidosis showed increasing CSF CHITO over time, suggesting that CSF CHITO reflects disease progression and a possible surrogate endpoint for future clinical trials with infantile GM1-gangliosidosis. In summary, these results support the use of CSF CHITO to diagnose between different disease phenotypes and as a valuable tool for monitoring disease progression in patients. These results necessitate the inclusion of CHITO as an exploratory biomarker for clinical trials.
Keywords: Chitotriosidase; GM1-gangliosidosis; GM2-gangliosidosis; Gaucher; Lysosomal diseases; Mucopolysaccharidosis.
© 2021 The Authors.
Figures




References
-
- van Eijk M., van Roomen C.P., Renkema G.H., Bussink A.P., Andrews L., Blommaart E.F., Sugar A., Verhoeven A.J., Boot R.G., Aerts J.M. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. - DOI - PubMed
LinkOut - more resources
Full Text Sources

