Effect of Kidney Transplantation on Accelerated Immunosenescence and Vascular Changes Induced by Chronic Kidney Disease
- PMID: 34646838
- PMCID: PMC8502880
- DOI: 10.3389/fmed.2021.705159
Effect of Kidney Transplantation on Accelerated Immunosenescence and Vascular Changes Induced by Chronic Kidney Disease
Abstract
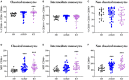
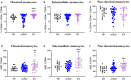
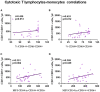
Kidney transplantation is the best option for patients with end-stage renal disease. Despite the improvement in cardiovascular burden (leading cause of mortality among patients with chronic kidney disease), cardiovascular adverse outcomes related to the inflammatory process remain a problem. Thus, the aim of the present study was to characterize the immune profile and microvesicles of patients who underwent transplantation. We investigated the lymphocyte phenotype (CD3, CD4, CD8, CD19, and CD56) and monocyte phenotype (CD14, CD16, CD86, and CD54) in peripheral blood, and endothelium-derived microvesicles (annexin V+CD31+CD41-) in plasma of patients with advanced chronic kidney disease (n = 40), patients with transplantation (n = 40), and healthy subjects (n = 18) recruited from the University Hospital "12 de Octubre" (Madrid, Spain). Patients with kidney transplantation had B-cell lymphopenia, an impairment in co-stimulatory (CD86) and adhesion (CD54) molecules in monocytes, and a reduction in endothelium-derived microvesicles in plasma. The correlations between those parameters explained the modifications in the expression of co-stimulatory and adhesion molecules in monocytes caused by changes in lymphocyte populations, as well as the increase in the levels of endothelial-derived microvesicles in plasma caused by changes in lymphocyte and monocytes populations. Immunosuppressive treatment could directly or indirectly induce those changes. Nevertheless, the particular characteristics of these cells may partly explain the persistence of cardiovascular and renal alterations in patients who underwent transplantation, along with the decrease in arteriosclerotic events compared with advanced chronic kidney disease. In conclusion, the expression of adhesion molecules by monocytes and endothelial-derived microvesicles is related to lymphocyte alterations in patients with kidney transplantation.
Keywords: chronic kidney disease; immunity; immunosenescence; microvesicles; renal transplantation.
Copyright © 2021 Ceprian, Valera, Caro, Yuste, Serroukh, González de Pablos, Oliva, Figuer, Praga, Alique, Ramirez, Morales and Carracedo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Ortiz A, Sanchez-Niño MD, Crespo-Barrio M, De-Sequera-Ortiz P, Fernández-Giráldez E, García-Maset R, et al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia. (2019) 39:29–34. 10.1016/j.nefro.2018.09.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

