Mitochondrial Regulation of Diabetic Kidney Disease
- PMID: 34646847
- PMCID: PMC8502854
- DOI: 10.3389/fmed.2021.745279
Mitochondrial Regulation of Diabetic Kidney Disease
Abstract
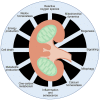
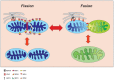
The role and nature of mitochondrial dysfunction in diabetic kidney disease (DKD) has been extensively studied. Yet, the molecular drivers of mitochondrial remodeling in DKD are poorly understood. Diabetic kidney cells exhibit a cascade of mitochondrial dysfunction ranging from changes in mitochondrial morphology to significant alterations in mitochondrial biogenesis, biosynthetic, bioenergetics and production of reactive oxygen species (ROS). How these changes individually or in aggregate contribute to progression of DKD remain to be fully elucidated. Nevertheless, because of the remarkable progress in our basic understanding of the role of mitochondrial biology and its dysfunction in DKD, there is great excitement on future targeted therapies based on improving mitochondrial function in DKD. This review will highlight the latest advances in understanding the nature of mitochondria dysfunction and its role in progression of DKD, and the development of mitochondrial targets that could be potentially used to prevent its progression.
Keywords: bioenergetics; diabetic kidney disease; mitochondria; mitochondrial dynamics; mitochondrial respiratory complexes; oxidative phosphorylation.
Copyright © 2021 Galvan, Mise and Danesh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

