Receptor-specific Ca2+ oscillation patterns mediated by differential regulation of P2Y purinergic receptors in rat hepatocytes
- PMID: 34646983
- PMCID: PMC8496176
- DOI: 10.1016/j.isci.2021.103139
Receptor-specific Ca2+ oscillation patterns mediated by differential regulation of P2Y purinergic receptors in rat hepatocytes
Abstract
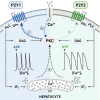
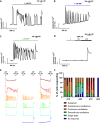
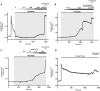
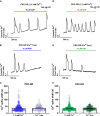
Extracellular agonists linked to inositol-1,4,5-trisphosphate (IP3) formation elicit cytosolic Ca2+ oscillations in many cell types, but despite a common signaling pathway, distinct agonist-specific Ca2+ spike patterns are observed. Using qPCR, we show that rat hepatocytes express multiple purinergic P2Y and P2X receptors (R). ADP acting through P2Y1R elicits narrow Ca2+ oscillations, whereas UTP acting through P2Y2R elicits broad Ca2+ oscillations, with composite patterns observed for ATP. P2XRs do not play a role at physiological agonist levels. The discrete Ca2+ signatures reflect differential effects of protein kinase C (PKC), which selectively modifies the falling phase of the Ca2+ spikes. Negative feedback by PKC limits the duration of P2Y1R-induced Ca2+ spikes in a manner that requires extracellular Ca2+. By contrast, P2Y2R is resistant to PKC negative feedback. Thus, the PKC leg of the bifurcated IP3 signaling pathway shapes unique Ca2+ oscillation patterns that allows for distinct cellular responses to different agonists.
Keywords: Biological sciences; Cell biology; Cellular physiology; Functional aspects of cell biology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Abbracchio M.P., Burnstock G., Boeynaems J.M., Barnard E.A., Boyer J.L., Kennedy C., Knight G.E., Fumagalli M., Gachet C., Jacobson K.A., Weisman G.A. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006;58:281–341. - PMC - PubMed
-
- Barrera N.P., Ormond S.J., Henderson R.M., Murrell-Lagnado R.D., Edwardson J.M. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J. Biol. Chem. 2005;280:10759–10765. - PubMed
-
- Bartlett P.J., Gaspers L.D., Pierobon N., Thomas A.P. Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium. 2014;55:306–316. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

