Bacillus Calmette-Guerin 's beneficial impact on glucose metabolism: evidence for broad based applications
- PMID: 34646988
- PMCID: PMC8501688
- DOI: 10.1016/j.isci.2021.103150
Bacillus Calmette-Guerin 's beneficial impact on glucose metabolism: evidence for broad based applications
Abstract
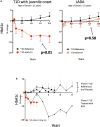
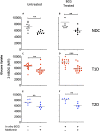
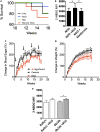
Bacillus Calmette-Guerin (BCG) vaccinations improve glycemic control in juvenile-onset Type I diabetes (T1D), an effect driven by restored sugar transport through aerobic glycolysis. In a pilot clinical trial, T1D, but not latent autoimmune diabetes of adults (LADA), exhibited lower blood sugars after multidose BCG. Using a glucose transport assay, monocytes from T1D subjects showed a large stimulation index with BCG exposures; LADA subjects showed minimal BCG-induced sugar responsiveness. Monocytes from T1D, type 2 diabetes (T2D), and non-diabetic controls (NDC) were all responsive in vitro to BCG by augmented sugar utilization. Adults with prior neonatal BCG vaccination show accelerated glucose transport decades later. Finally, in vivo experiments with the NOD mouse (a T1D model) and obese db/db mice (a T2D model) confirm BCG's blood-sugar-lowering and accelerated glucose metabolism with sufficient dosing. Our results suggest that BCG's benefits for glucose metabolism may be broadly applicable to T1D and T2D, but less to LADA.
Keywords: Human metabolism; Immunology.
© 2021 The Authors.
Conflict of interest statement
All authors declare no competing interests.
Figures



Similar articles
-
Bacille Calmette Guerin (BCG) and prevention of types 1 and 2 diabetes: Results of two observational studies.PLoS One. 2023 Jan 20;18(1):e0276423. doi: 10.1371/journal.pone.0276423. eCollection 2023. PLoS One. 2023. PMID: 36662841 Free PMC article.
-
The spleen assumes a major role in blood glucose regulation in type 1 diabetes patients treated with BCG.Sci Rep. 2024 Jul 30;14(1):17611. doi: 10.1038/s41598-024-67905-x. Sci Rep. 2024. PMID: 39080423 Free PMC article.
-
Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations.NPJ Vaccines. 2018 Jun 21;3:23. doi: 10.1038/s41541-018-0062-8. eCollection 2018. NPJ Vaccines. 2018. PMID: 29951281 Free PMC article.
-
BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism.Trends Endocrinol Metab. 2019 Feb;30(2):80-92. doi: 10.1016/j.tem.2018.11.006. Epub 2018 Dec 29. Trends Endocrinol Metab. 2019. PMID: 30600132 Review.
-
Significance of Bacillus Calmette-Guerin (BCG) vaccine intervention for patients with Type 1 Diabetes (T1D): A systematic review and meta-analysis.Diabetes Metab Syndr. 2024 Aug;18(8):103102. doi: 10.1016/j.dsx.2024.103102. Epub 2024 Aug 12. Diabetes Metab Syndr. 2024. PMID: 39173532
Cited by
-
Repurposing peripheral immunocytes of Bacillus Calmette Guerin-vaccinated melanoma patients to reveal preventive Alzheimer's disease mechanisms, possibly via the unfolded protein response.J Alzheimers Dis Rep. 2025 Feb 10;9:25424823241309664. doi: 10.1177/25424823241309664. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40034523 Free PMC article.
-
Specific and non-specific effects of Mycobacterium bovis BCG vaccination in dairy calves.Front Vet Sci. 2023 Oct 6;10:1278329. doi: 10.3389/fvets.2023.1278329. eCollection 2023. Front Vet Sci. 2023. PMID: 37869491 Free PMC article.
-
Bacille Calmette Guerin (BCG) and prevention of types 1 and 2 diabetes: Results of two observational studies.PLoS One. 2023 Jan 20;18(1):e0276423. doi: 10.1371/journal.pone.0276423. eCollection 2023. PLoS One. 2023. PMID: 36662841 Free PMC article.
-
BCG Vaccination Suppresses Glucose Intolerance Progression in High-Fat-Diet-Fed C57BL/6 Mice.Medicina (Kaunas). 2024 May 25;60(6):866. doi: 10.3390/medicina60060866. Medicina (Kaunas). 2024. PMID: 38929483 Free PMC article.
-
The spleen assumes a major role in blood glucose regulation in type 1 diabetes patients treated with BCG.Sci Rep. 2024 Jul 30;14(1):17611. doi: 10.1038/s41598-024-67905-x. Sci Rep. 2024. PMID: 39080423 Free PMC article.
References
-
- Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. Published online 2011/06/16. - DOI - PubMed
-
- Aaby P., Vessari H., Nielsen J., Maleta K., Benn C.S., Jensen H., Ashorn P. Sex differential effects of routine immunizations and childhood survival in rural Malawi. Pediatr. Infect Dis. J. 2006;25:721–727. doi: 10.1097/01.inf.0000227829.64686.ae. Published online 2006/07/29. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

