The spatial form periosteal-bone complex promotes bone regeneration by coordinating macrophage polarization and osteogenic-angiogenic events
- PMID: 34647005
- PMCID: PMC8495177
- DOI: 10.1016/j.mtbio.2021.100142
The spatial form periosteal-bone complex promotes bone regeneration by coordinating macrophage polarization and osteogenic-angiogenic events
Abstract
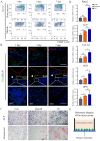
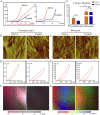
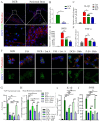
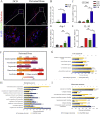
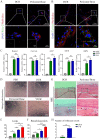
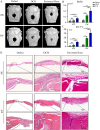
Bone defects associated with soft tissue injuries are an important cause of deformity that threatens people's health and quality of life. Although bone substitutes have been extensively explored, effective biomaterials that can coordinate early inflammation regulation and subsequent repair events are still lacking. We prepared a spatial form periosteal bone extracellular matrix (ECM) scaffold, which has advantages in terms of low immunogenicity, good retention of bioactive ingredients, and a natural spatial structure. The periosteal bone ECM scaffold with the relatively low-stiffness periosteum (41.6 ± 3.7 kPa) could inhibit iNOS and IL-1β expression, which might be related to actin-mediated YAP translocation. It also helped to promote CD206 expression with the potential influence of proteins related to immune regulation. Moreover, the scaffold combined the excellent properties of decalcified bone and periosteum, promoted the formation of blood vessels, and good osteogenic differentiation (RUNX2, Col 1α1, ALP, OPN, and OCN), and achieved good repair of a cranial defect in rats. This scaffold, with its natural structural and biological advantages, provides a new idea for bone healing treatment that is aligned with bone physiology.
Keywords: Angiogenesis; Bone healing; Macrophage polarization; Osteogenesis; Periosteal-bone complex; Stiffness.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis.Biomaterials. 2020 Jan;227:119552. doi: 10.1016/j.biomaterials.2019.119552. Epub 2019 Oct 18. Biomaterials. 2020. PMID: 31670079
-
Spatiotemporal Immunomodulation and Biphasic Osteo-Vascular Aligned Electrospun Membrane for Diabetic Periosteum Regeneration.Adv Sci (Weinh). 2023 Dec;10(36):e2302874. doi: 10.1002/advs.202302874. Epub 2023 Nov 16. Adv Sci (Weinh). 2023. PMID: 37973554 Free PMC article.
-
[Repair of calvarial defect using a tissue-engineered bone with simvastatin-loaded β-tricalcium phosphate scaffold and adipose derived stem cells in rabbits].Shanghai Kou Qiang Yi Xue. 2013 Aug;22(4):361-7. Shanghai Kou Qiang Yi Xue. 2013. PMID: 24100891 Chinese.
-
Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats.Stem Cell Res Ther. 2017 Jun 5;8(1):134. doi: 10.1186/s13287-017-0592-4. Stem Cell Res Ther. 2017. PMID: 28583167 Free PMC article.
-
The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration.Acta Biomater. 2017 Oct 1;61:217-232. doi: 10.1016/j.actbio.2017.08.015. Epub 2017 Aug 12. Acta Biomater. 2017. PMID: 28807800
Cited by
-
Recombinant humanized collagen remodels endometrial immune microenvironment of chronic endometritis through macrophage immunomodulation.Regen Biomater. 2023 Apr 3;10:rbad033. doi: 10.1093/rb/rbad033. eCollection 2023. Regen Biomater. 2023. PMID: 37122820 Free PMC article.
-
3D cryo-printed hierarchical porous scaffolds provide immobilization of surface-functionalized sleep-inspired small extracellular vesicles: synergistic therapeutic strategies for vascularized bone regeneration based on macrophage phenotype modulation and angiogenesis-osteogenesis coupling.J Nanobiotechnology. 2024 Dec 19;22(1):764. doi: 10.1186/s12951-024-02977-5. J Nanobiotechnology. 2024. PMID: 39695679 Free PMC article.
-
Hippo Signaling Pathway Involvement in Osteopotential Regulation of Murine Bone Marrow Cells Under Simulated Microgravity.Cells. 2024 Nov 19;13(22):1921. doi: 10.3390/cells13221921. Cells. 2024. PMID: 39594669 Free PMC article.
-
Hippo-YAP/TAZ signaling in osteogenesis and macrophage polarization: Therapeutic implications in bone defect repair.Genes Dis. 2023 Jan 16;10(6):2528-2539. doi: 10.1016/j.gendis.2022.12.012. eCollection 2023 Nov. Genes Dis. 2023. PMID: 37554194 Free PMC article. Review.
-
Personalized PLGA/BCL Scaffold with Hierarchical Porous Structure Resembling Periosteum-Bone Complex Enables Efficient Repair of Bone Defect.Adv Sci (Weinh). 2024 Sep;11(35):e2401589. doi: 10.1002/advs.202401589. Epub 2024 Jul 17. Adv Sci (Weinh). 2024. PMID: 39018263 Free PMC article.
References
-
- Bai L., Liu Y., Du Z., Weng Z., Yao W., Zhang X., Huang X., Yao X., Crawford R., Hang R., Huang D., Tang B., Xiao Y. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018;76:344–358. doi: 10.1016/j.actbio.2018.06.023. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

