Prevention of vascular calcification by the endogenous chromogranin A-derived mediator that inhibits osteogenic transdifferentiation
- PMID: 34647168
- PMCID: PMC8514386
- DOI: 10.1007/s00395-021-00899-z
Prevention of vascular calcification by the endogenous chromogranin A-derived mediator that inhibits osteogenic transdifferentiation
Abstract
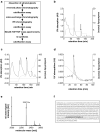
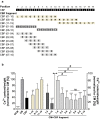
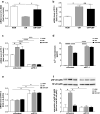
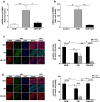
The adrenal glands participate in cardiovascular (CV) physiology and the pathophysiology of CV diseases through their effects on sodium and water metabolism, vascular tone and cardiac function. In the present study, we identified a new adrenal compound controlling mesenchymal cell differentiation that regulates osteoblastic differentiation in the context of vascular calcification. This peptide was named the "calcification blocking factor" (CBF) due to its protective effect against vascular calcification and is released from chromogranin A via enzymatic cleavage by calpain 1 and kallikrein. CBF reduced the calcium content of cells and thoracic aortic rings under calcifying culture conditions, as well as in aortas from animals treated with vitamin D and nicotine (VDN animals). Furthermore, CBF prevented vascular smooth muscle cell (VSMC) transdifferentiation into osteoblast-like cells within the vascular wall via the sodium-dependent phosphate transporter PIT-1 and by inhibition of NF-κB activation and the subsequent BMP2/p-SMAD pathway. Pulse pressure, a marker of arterial stiffness, was significantly decreased in VDN animals treated with CBF. In line with our preclinical data, CBF concentration is significantly reduced in diseases characterized by increased calcification, as shown in patients with chronic kidney disease. In preparation for clinical translation, the active site of the native 19-AS long native CBF was identified as EGQEEEED. In conclusion, we have identified the new peptide CBF, which is secreted from the adrenal glands and might prevent vascular calcification by inhibition of osteogenic transdifferentiation. The anti-calcific effects of CBF and short active site may therefore promote the development of new tools for the prevention and/or treatment of vascular calcification.
Keywords: Adrenal glands; Cardiorenal syndrome; Vascular calcification; Vascular smooth muscle cell transdifferentiation.
© 2021. The Author(s).
Conflict of interest statement
All the authors declare that they have no conflict of interest.
Figures




References
-
- Balderman JA, Lee HY, Mahoney CE, Handy DE, White K, Annis S, Lebeche D, Hajjar RJ, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc. 2012;1:e003905. doi: 10.1161/JAHA.112.003905. - DOI - PMC - PubMed
-
- Baulmann J, Schillings U, Rickert S, Uen S, Dusing R, Illyes M, Cziraki A, Nickering G, Mengden T. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. 2008;26:523–528. doi: 10.1097/HJH.0b013e3282f314f7. - DOI - PubMed
-
- Bokui N, Otani T, Igarashi K, Kaku J, Oda M, Nagaoka T, Seno M, Tatematsu K, Okajima T, Matsuzaki T, Ting K, Tanizawa K, Kuroda S. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008;582:365–371. doi: 10.1016/j.febslet.2007.12.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

