The conundrum of pharyngeal teeth origin: the role of germ layers, pouches, and gill slits
- PMID: 34647411
- PMCID: PMC9293187
- DOI: 10.1111/brv.12805
The conundrum of pharyngeal teeth origin: the role of germ layers, pouches, and gill slits
Abstract
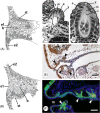
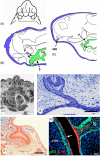
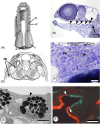
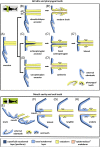
There are several competing hypotheses on tooth origins, with discussions eventually settling in favour of an 'outside-in' scenario, in which internal odontodes (teeth) derived from external odontodes (skin denticles) in jawless vertebrates. The evolution of oral teeth from skin denticles can be intuitively understood from their location at the mouth entrance. However, the basal condition for jawed vertebrates is arguably to possess teeth distributed throughout the oropharynx (i.e. oral and pharyngeal teeth). As skin denticle development requires the presence of ectoderm-derived epithelium and of mesenchyme, it remains to be answered how odontode-forming skin epithelium, or its competence, were 'transferred' deep into the endoderm-covered oropharynx. The 'modified outside-in' hypothesis for tooth origins proposed that this transfer was accomplished through displacement of odontogenic epithelium, that is ectoderm, not only through the mouth, but also via any opening (e.g. gill slits) that connects the ectoderm to the epithelial lining of the pharynx (endoderm). This review explores from an evolutionary and from a developmental perspective whether ectoderm plays a role in (pharyngeal) tooth and denticle formation. Historic and recent studies on tooth development show that the odontogenic epithelium (enamel organ) of oral or pharyngeal teeth can be of ectodermal, endodermal, or of mixed ecto-endodermal origin. Comprehensive data are, however, only available for a few taxa. Interestingly, in these taxa, the enamel organ always develops from the basal layer of a stratified epithelium that is at least bilayered. In zebrafish, a miniaturised teleost that only retains pharyngeal teeth, an epithelial surface layer with ectoderm-like characters is required to initiate the formation of an enamel organ from the basal, endodermal epithelium. In urodele amphibians, the bilayered epithelium is endodermal, but the surface layer acquires ectodermal characters, here termed 'epidermalised endoderm'. Furthermore, ectoderm-endoderm contacts at pouch-cleft boundaries (i.e. the prospective gill slits) are important for pharyngeal tooth initiation, even if the influx of ectoderm via these routes is limited. A balance between sonic hedgehog and retinoic acid signalling could operate to assign tooth-initiating competence to the endoderm at the level of any particular pouch. In summary, three characters are identified as being required for pharyngeal tooth formation: (i) pouch-cleft contact, (ii) a stratified epithelium, of which (iii) the apical layer adopts ectodermal features. These characters delimit the area in which teeth can form, yet cannot alone explain the distribution of teeth over the different pharyngeal arches. The review concludes with a hypothetical evolutionary scenario regarding the persisting influence of ectoderm on pharyngeal tooth formation. Studies on basal osteichthyans with less-specialised types of early embryonic development will provide a crucial test for the potential role of ectoderm in pharyngeal tooth formation and for the 'modified outside-in' hypothesis of tooth origins.
Keywords: dermal denticles; ectoderm; endoderm; oropharynx; periderm; pharyngeal denticles; pharyngeal pouches; tooth development.
© 2021 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures






Similar articles
-
Multiple epithelia are required to develop teeth deep inside the pharynx.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11503-11512. doi: 10.1073/pnas.2000279117. Epub 2020 May 12. Proc Natl Acad Sci U S A. 2020. PMID: 32398375 Free PMC article.
-
Periderm fate and independence of tooth formation are conserved across osteichthyans.Evodevo. 2024 Oct 3;15(1):13. doi: 10.1186/s13227-024-00232-4. Evodevo. 2024. PMID: 39363199 Free PMC article.
-
Evolutionary and developmental origins of the vertebrate dentition.J Anat. 2009 Apr;214(4):465-76. doi: 10.1111/j.1469-7580.2009.01053.x. J Anat. 2009. PMID: 19422425 Free PMC article. Review.
-
Dual epithelial origin of vertebrate oral teeth.Nature. 2008 Oct 9;455(7214):795-8. doi: 10.1038/nature07304. Epub 2008 Sep 14. Nature. 2008. PMID: 18794902
-
Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms.Biol Rev Camb Philos Soc. 2005 May;80(2):303-45. doi: 10.1017/s1464793104006682. Biol Rev Camb Philos Soc. 2005. PMID: 15921053 Review.
Cited by
-
Modulation of tooth regeneration through opposing responses to Wnt and BMP signals in teleosts.Development. 2023 Dec 1;150(23):dev202168. doi: 10.1242/dev.202168. Epub 2023 Dec 7. Development. 2023. PMID: 38059590 Free PMC article.
-
Complex enameloid microstructure of †Ischyrhiza mira rostral denticles.J Anat. 2022 Sep;241(3):616-627. doi: 10.1111/joa.13676. Epub 2022 Apr 20. J Anat. 2022. PMID: 35445396 Free PMC article.
-
Morphogenesis of pteraspid heterostracan oral plates and the evolutionary origin of teeth.R Soc Open Sci. 2024 Dec 18;11(12):240836. doi: 10.1098/rsos.240836. eCollection 2024 Dec. R Soc Open Sci. 2024. PMID: 39698157 Free PMC article.
-
Interference with the retinoic acid signalling pathway inhibits the initiation of teeth and caudal primary scales in the small-spotted catshark Scyliorhinus canicula.PeerJ. 2023 Sep 6;11:e15896. doi: 10.7717/peerj.15896. eCollection 2023. PeerJ. 2023. PMID: 37692112 Free PMC article.
-
The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration.Int J Mol Sci. 2023 Sep 5;24(18):13692. doi: 10.3390/ijms241813692. Int J Mol Sci. 2023. PMID: 37761995 Free PMC article.
References
-
- Adams, A. E. (1924). An experimental study of the development of the mouth in the amphibian embryo. Journal of Experimental Zoology 40, 311–379.
-
- Adams, A. E. (1931). Some effects of removal of endoderm from the mouth region of early Amblystoma punctatum embryos. Journal of Experimental Zoology 58, 147–163.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

