Virologic Features of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children
- PMID: 34647601
- PMCID: PMC8643403
- DOI: 10.1093/infdis/jiab509
Virologic Features of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children
Abstract
Background: Data on pediatric coronavirus disease 2019 (COVID-19) has lagged behind adults throughout the pandemic. An understanding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral dynamics in children would enable data-driven public health guidance.
Methods: Respiratory swabs were collected from children with COVID-19. Viral load was quantified by reverse-transcription polymerase chain reaction (RT-PCR); viral culture was assessed by direct observation of cytopathic effects and semiquantitative viral titers. Correlations with age, symptom duration, and disease severity were analyzed. SARS-CoV-2 whole genome sequences were compared with contemporaneous sequences.
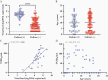
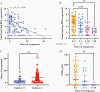
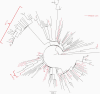
Results: One hundred ten children with COVID-19 (median age, 10 years [range, 2 weeks-21 years]) were included in this study. Age did not impact SARS-CoV-2 viral load. Children were most infectious within the first 5 days of illness, and severe disease did not correlate with increased viral loads. Pediatric SARS-CoV-2 sequences were representative of those in the community and novel variants were identified.
Conclusions: Symptomatic and asymptomatic children can carry high quantities of live, replicating SARS-CoV-2, creating a potential reservoir for transmission and evolution of genetic variants. As guidance around social distancing and masking evolves following vaccine uptake in older populations, a clear understanding of SARS-CoV-2 infection dynamics in children is critical for rational development of public health policies and vaccination strategies to mitigate the impact of COVID-19.
Keywords: SARS-CoV-2; pediatric COVID-19; viral dynamics.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Update of
-
Virologic features of SARS-CoV-2 infection in children.medRxiv [Preprint]. 2021 Aug 17:2021.05.30.21258086. doi: 10.1101/2021.05.30.21258086. medRxiv. 2021. Update in: J Infect Dis. 2021 Dec 1;224(11):1821-1829. doi: 10.1093/infdis/jiab509. PMID: 34124714 Free PMC article. Updated. Preprint.
Comment in
-
Coronavirus Disease 2019 and Children.J Infect Dis. 2021 Dec 1;224(11):1807-1809. doi: 10.1093/infdis/jiab511. J Infect Dis. 2021. PMID: 34647593 Free PMC article. No abstract available.
References
-
- American Academy of Pediatrics. Children and COVID-19: state-level data report. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infect.... Accessed 22 July 2021.
-
- Massachusetts Department of Public Health. COVID-19 response reporting. https://www.mass.gov/info-details/covid-19-response-reporting. Accessed 22 July 2021.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

