Rapid Analysis of ADP-Ribosylation Dynamics and Site-Specificity Using TLC-MALDI
- PMID: 34647721
- PMCID: PMC8609518
- DOI: 10.1021/acschembio.1c00542
Rapid Analysis of ADP-Ribosylation Dynamics and Site-Specificity Using TLC-MALDI
Abstract
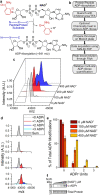
Poly(ADP-ribose) polymerases, PARPs, transfer ADP-ribose onto target proteins from nicotinamide adenine dinucleotide (NAD+). Current mass spectrometric analytical methods require proteolysis of target proteins, limiting the study of dynamic ADP-ribosylation on contiguous proteins. Herein, we present a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) method that facilitates multisite analysis of ADP-ribosylation. We observe divergent ADP-ribosylation dynamics for the catalytic domains of PARPs 14 and 15, with PARP15 modifying more sites on itself (+3-4 ADP-ribose) than the closely related PARP14 protein (+1-2 ADP-ribose)─despite similar numbers of potential modification sites. We identify, for the first time, a minimal peptide fragment (18 amino-acids) that is preferentially modified by PARP14. Finally, we demonstrate through mutagenesis and chemical treatment with hydroxylamine that PARPs 14/15 prefer acidic residues. Our results highlight the utility of MALDI-TOF in the analysis of PARP target modifications and in elucidating the biochemical mechanism governing PARP target selection.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

