Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial
- PMID: 34647988
- PMCID: PMC8517885
- DOI: 10.1001/jamaoncol.2021.4761
Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial
Erratum in
-
Error in Abstract and Methods.JAMA Oncol. 2024 Oct 1;10(10):1443. doi: 10.1001/jamaoncol.2022.0062. JAMA Oncol. 2024. PMID: 35201294 Free PMC article. No abstract available.
-
Error in Results and Table 1.JAMA Oncol. 2022 Sep 1;8(9):1359. doi: 10.1001/jamaoncol.2022.4366. JAMA Oncol. 2022. PMID: 36107172 Free PMC article. No abstract available.
Abstract
Importance: Metastatic non-small cell lung cancer (mNSCLC) with EGFR exon 20 insertion (EGFRex20ins) mutations is associated with a poor prognosis. Mobocertinib is an oral tyrosine kinase inhibitor designed to selectively target EGFRex20ins mutations.
Objective: To evaluate treatment outcomes and safety of mobocertinib in patients with previously treated EGFRex20ins-positive mNSCLC.
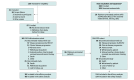
Design, setting, and participants: This 3-part, open-label, phase 1/2 nonrandomized clinical trial with dose-escalation/dose-expansion cohorts (28 sites in the US) and a single-arm extension cohort (EXCLAIM; 40 sites in Asia, Europe, and North America) was conducted between June 2016 and November 2020 (data cutoff date). The primary analysis populations were the platinum-pretreated patients (PPP) cohort and the EXCLAIM cohort. The PPP cohort included 114 patients with platinum-pretreated EGFRex20ins-positive mNSCLC who received mobocertinib 160 mg once daily from the dose-escalation (n = 6), dose-expansion (n = 22), and EXCLAIM (n = 86) cohorts. The EXCLAIM cohort included 96 patients with previously treated EGFRex20ins-positive mNSCLC (10 were not platinum pretreated and thus were excluded from the PPP cohort).
Interventions: Mobocertinib 160 mg once daily.
Main outcomes and measures: The primary end point of the PPP and EXCLAIM cohorts was confirmed objective response rate (ORR) assessed by independent review committee (IRC). Secondary end points included confirmed ORR by investigator, duration of response, progression-free survival, overall survival, and safety.
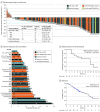
Results: Among the PPP (n = 114) and EXCLAIM (n = 96) cohorts, the median (range) age was 60 (27-84) and 59 (27-80) years, respectively; most patients were women (75 [66%] and 62 [65%], respectively) and of Asian race (68 [60%] and 66 [69%], respectively). At data cutoff, median follow-up was 14.2 months in the PPP cohort (median 2 prior anticancer regimens; 40 [35%] had baseline brain metastases), with confirmed ORR of 28% (95% CI, 20%-37%) by IRC assessment and 35% (95% CI, 26%-45%) by investigator assessment; median duration of response by IRC assessment was 17.5 months (95% CI, 7.4-20.3). Median progression-free survival by IRC assessment was 7.3 months (95% CI, 5.5-9.2). Median overall survival was 24.0 months (95% CI, 14.6-28.8). In the EXCLAIM cohort, median follow-up was 13.0 months, with confirmed ORR by IRC assessment of 25% (95% CI, 17%-35%) and by investigator assessment of 32% (95% CI, 23%-43%). The most common treatment-related adverse events were diarrhea and rash.
Conclusions and relevance: In this open-label, phase 1/2 nonrandomized clinical trial, mobocertinib was associated with clinically meaningful benefit in patients with previously treated EGFRex20ins-positive mNSCLC, with a manageable safety profile.
Trial registration: ClinicalTrials.gov Identifier: NCT02716116.
Conflict of interest statement
Figures
Similar articles
-
First-Line Mobocertinib Versus Platinum-Based Chemotherapy in Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer in the Phase III EXCLAIM-2 Trial.J Clin Oncol. 2025 May;43(13):1553-1563. doi: 10.1200/JCO-24-01269. Epub 2025 Jan 29. J Clin Oncol. 2025. PMID: 39879577 Free PMC article. Clinical Trial.
-
Mobocertinib: Mechanism of action, clinical, and translational science.Clin Transl Sci. 2024 Mar;17(3):e13766. doi: 10.1111/cts.13766. Clin Transl Sci. 2024. PMID: 38511563 Free PMC article. Review.
-
Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial.Cancer Discov. 2021 Jul;11(7):1688-1699. doi: 10.1158/2159-8290.CD-20-1598. Epub 2021 Feb 25. Cancer Discov. 2021. PMID: 33632775 Free PMC article. Clinical Trial.
-
Clinical Utility of Mobocertinib in the Treatment of NSCLC - Patient Selection and Reported Outcomes.Onco Targets Ther. 2023 Jul 11;16:559-569. doi: 10.2147/OTT.S374489. eCollection 2023. Onco Targets Ther. 2023. PMID: 37456145 Free PMC article. Review.
-
Efficacy of Mobocertinib and Amivantamab in Patients With Advanced Non-Small Cell Lung Cancer With EGFR Exon 20 Insertions Previously Treated With Platinum-Based Chemotherapy: An Indirect Treatment Comparison.Clin Lung Cancer. 2024 May;25(3):e145-e152.e3. doi: 10.1016/j.cllc.2023.11.011. Epub 2023 Dec 4. Clin Lung Cancer. 2024. PMID: 38114357 Clinical Trial.
Cited by
-
Drug development and evidence for lung cancer targeted therapy in Eastern Asia.Lancet Reg Health West Pac. 2024 Jul 8;49:101090. doi: 10.1016/j.lanwpc.2024.101090. eCollection 2024 Aug. Lancet Reg Health West Pac. 2024. PMID: 39381018 Free PMC article. Review.
-
Long-term survival after stereotactic body radiotherapy combined with immunotherapy plus anti-angiogenesis therapy in patients with advanced non-small cell lung cancer and EGFR exon 20 insertion mutation: a report of two cases.Transl Lung Cancer Res. 2023 Nov 30;12(11):2330-2341. doi: 10.21037/tlcr-23-542. Epub 2023 Nov 20. Transl Lung Cancer Res. 2023. PMID: 38090524 Free PMC article.
-
EGFR exon 20 insertion variants A763_Y764insFQEA and D770delinsGY confer favorable sensitivity to currently approved EGFR-specific tyrosine kinase inhibitors.Front Pharmacol. 2022 Nov 8;13:984503. doi: 10.3389/fphar.2022.984503. eCollection 2022. Front Pharmacol. 2022. PMID: 36425568 Free PMC article.
-
Rare molecular subtypes of lung cancer.Nat Rev Clin Oncol. 2023 Apr;20(4):229-249. doi: 10.1038/s41571-023-00733-6. Epub 2023 Feb 20. Nat Rev Clin Oncol. 2023. PMID: 36806787 Free PMC article. Review.
-
Treatment Strategies for Non-Small Cell Lung Cancer Harboring Common and Uncommon EGFR Mutations: Drug Sensitivity Based on Exon Classification, and Structure-Function Analysis.Cancers (Basel). 2022 May 20;14(10):2519. doi: 10.3390/cancers14102519. Cancers (Basel). 2022. PMID: 35626123 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

