Genetic inhibition of nuclear factor of activated T-cell c2 prevents atrial fibrillation in CREM transgenic mice
- PMID: 34648001
- PMCID: PMC9586567
- DOI: 10.1093/cvr/cvab325
Genetic inhibition of nuclear factor of activated T-cell c2 prevents atrial fibrillation in CREM transgenic mice
Abstract
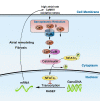
Aims: Abnormal intracellular calcium (Ca2+) handling contributes to the progressive nature of atrial fibrillation (AF), the most common sustained cardiac arrhythmia. Evidence in mouse models suggests that activation of the nuclear factor of activated T-cell (NFAT) signalling pathway contributes to atrial remodelling. Our aim was to determine the role of NFATc2 in AF in humans and mouse models.
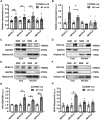
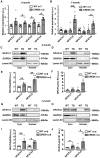
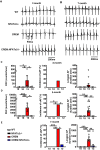
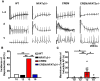
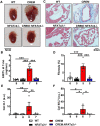
Methods and results: Expression levels of NFATc1-c4 isoforms were assessed by quantitative reverse transcription-polymerase chain reaction in right atrial appendages from patients with chronic AF (cAF). NFATc1 and NFATc2 mRNA levels were elevated in cAF patients compared with those in normal sinus rhythm (NSR). Western blotting revealed increased cytosolic and nuclear levels of NFATc2 in AF patients. Similar findings were obtained in CREM-IbΔC-X transgenic (CREM) mice, a model of progressive AF. Telemetry ECG recordings revealed age-dependent spontaneous AF in CREM mice, which was prevented by NFATc2 knockout in CREM:NFATc2-/- mice. Programmed electrical stimulation revealed that CREM:NFATc2-/- mice lacked an AF substrate. Morphometric analysis and histology revealed increased atrial weight and atrial fibrosis in CREM mice compared with wild-type controls, which was reversed in CREM:NFATc2-/- mice. Confocal microscopy showed an increased Ca2+ spark frequency despite a reduced sarcoplasmic reticulum (SR) Ca2+ load in CREM mice compared with controls, whereas these abnormalities were normalized in CREM:NFATc2-/- mice. Western blotting revealed that genetic inhibition of Ca2+/calmodulin-dependent protein kinase II-mediated phosphorylation of S2814 on ryanodine receptor type 2 (RyR2) in CREM:RyR2-S2814A mice suppressed NFATc2 activation observed in CREM mice, suggesting that NFATc2 is activated by excessive SR Ca2+ leak via RyR2. Finally, chromatin immunoprecipitation sequencing from AF patients identified Ras and EF-hand domain-containing protein (Rasef) as a direct target of NFATc2-mediated transcription.
Conclusion: Our findings reveal activation of the NFAT signalling pathway in patients of Chinese and European descent. NFATc2 knockout prevents the progression of AF in the CREM mouse model.
Keywords: Atrial fibrillation; Atrial remodelling; Calcium handling; NFAT; RASEF.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: X.H.T.W. is a co-founder and Scientific Advisory Board member of Elex Biotech, a drug development company focused on novel compounds for the cardiac arrhythmia disorders and heart failure. All other authors declared no conflict of interest.
Figures


Similar articles
-
Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model.Circulation. 2014 Mar 25;129(12):1276-1285. doi: 10.1161/CIRCULATIONAHA.113.006611. Epub 2014 Jan 7. Circulation. 2014. PMID: 24398018 Free PMC article.
-
Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice.Circ Res. 2012 Feb 3;110(3):465-70. doi: 10.1161/CIRCRESAHA.111.253229. Epub 2011 Dec 8. Circ Res. 2012. PMID: 22158709 Free PMC article.
-
Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation.Circulation. 2012 May 1;125(17):2059-70. doi: 10.1161/CIRCULATIONAHA.111.067306. Epub 2012 Mar 28. Circulation. 2012. PMID: 22456474 Free PMC article.
-
Calcium-mediated cellular triggered activity in atrial fibrillation.J Physiol. 2017 Jun 15;595(12):4001-4008. doi: 10.1113/JP273048. Epub 2017 Mar 22. J Physiol. 2017. PMID: 28181690 Free PMC article. Review.
-
Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation.Trends Cardiovasc Med. 2010 Jan;20(1):30-4. doi: 10.1016/j.tcm.2010.03.004. Trends Cardiovasc Med. 2010. PMID: 20685575 Free PMC article. Review.
Cited by
-
Decreased METTL3 in atrial myocytes promotes atrial fibrillation.Europace. 2025 Feb 5;27(2):euaf021. doi: 10.1093/europace/euaf021. Europace. 2025. PMID: 39991872 Free PMC article.
-
Cytoplasmic and nuclear NFATc3 cooperatively contributes to vascular smooth muscle cell dysfunction and drives aortic aneurysm and dissection.Acta Pharm Sin B. 2025 Jul;15(7):3663-3684. doi: 10.1016/j.apsb.2025.05.016. Epub 2025 May 21. Acta Pharm Sin B. 2025. PMID: 40698138 Free PMC article.
-
Maintenance and Reversibility of Paroxysmal Atrial Fibrillation in JDP2 Overexpressing Mice.Cells. 2025 Jul 15;14(14):1079. doi: 10.3390/cells14141079. Cells. 2025. PMID: 40710332 Free PMC article.
-
Immune cells and arrhythmias.Cardiovasc Res. 2025 Apr 29;121(3):382-395. doi: 10.1093/cvr/cvaf017. Cardiovasc Res. 2025. PMID: 39937651 Review.
-
A cardioimmunologist's toolkit: genetic tools to dissect immune cells in cardiac disease.Nat Rev Cardiol. 2022 Jun;19(6):395-413. doi: 10.1038/s41569-022-00701-0. Epub 2022 May 6. Nat Rev Cardiol. 2022. PMID: 35523863 Review.
References
-
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC.. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–953. - PubMed
-
- Calkins H. When it comes to defining the outcomes of catheter ablation of atrial fibrillation, an implantable monitor is a great place to start. Circulation 2019;140:1789–1791. - PubMed
-
- Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH.. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 2009;119:1940–1951. - PMC - PubMed
-
- Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT.. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014;129:1276–1285. - PMC - PubMed
-
- Muller FU, Lewin G, Baba HA, Boknik P, Fabritz L, Kirchhefer U, Kirchhof P, Loser K, Matus M, Neumann J, Riemann B, Schmitz W.. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J Biol Chem 2005;280:6906–6914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

