Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19
- PMID: 34648357
- PMCID: PMC11000440
- DOI: 10.1126/scitranslmed.abj7790
Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19
Abstract
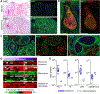
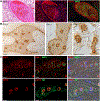
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by respiratory distress, multiorgan dysfunction, and, in some cases, death. The pathological mechanisms underlying COVID-19 respiratory distress and the interplay with aggravating risk factors have not been fully defined. Lung autopsy samples from 18 patients with fatal COVID-19, with symptom onset-to-death times ranging from 3 to 47 days, and antemortem plasma samples from 6 of these cases were evaluated using deep sequencing of SARS-CoV-2 RNA, multiplex plasma protein measurements, and pulmonary gene expression and imaging analyses. Prominent histopathological features in this case series included progressive diffuse alveolar damage with excessive thrombosis and late-onset pulmonary tissue and vascular remodeling. Acute damage at the alveolar-capillary barrier was characterized by the loss of surfactant protein expression with injury to alveolar epithelial cells, endothelial cells, respiratory epithelial basal cells, and defective tissue repair processes. Other key findings included impaired clot fibrinolysis with increased concentrations of plasma and lung plasminogen activator inhibitor-1 and modulation of cellular senescence markers, including p21 and sirtuin-1, in both lung epithelial and endothelial cells. Together, these findings further define the molecular pathological features underlying the pulmonary response to SARS-CoV-2 infection and provide important insights into signaling pathways that may be amenable to therapeutic intervention.
Conflict of interest statement
S.J.O. has received funding from Agenus, Amgen, Biothera, Bristol Myers Squibb, Exicure, Genocea, Incyte, Merck, Ultimovacs, and Viralytics. S.J.O. has consulted for Biothera, Bristol Myers Squibb, BioNTech, Exicure, Immunsys, and Merck. K. Sadtler is an inventor on a provisional U.S. patent application no. 63/092,350 entitled “Antibody specific for SARS-CoV-2 receptor binding domain and therapeutic methods.” The other authors declare that they have no competing interests.
Figures





References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324, 782–793 (2020). - PubMed
-
- Sauter JL, Baine MK, Butnor KJ, Buonocore DJ, Chang JC, Jungbluth AA, Szabolcs MJ, Morjaria S, Mount SL, Rekhtman N, Selbs E, Sheng ZM, Xiao Y, Kleiner DE, Pittaluga S, Taubenberger JK, Rapkiewicz AV, Travis WD, Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology 77, 915–925 (2020). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

