Deconstructing virus condensation
- PMID: 34648608
- PMCID: PMC8516229
- DOI: 10.1371/journal.ppat.1009926
Deconstructing virus condensation
Abstract
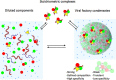
Viruses have evolved precise mechanisms for using the cellular physiological pathways for their perpetuation. These virus-driven biochemical events must be separated in space and time from those of the host cell. In recent years, granular structures, known for over a century for rabies virus, were shown to host viral gene function and were named using terms such as viroplasms, replication sites, inclusion bodies, or viral factories (VFs). More recently, these VFs were shown to be liquid-like, sharing properties with membrane-less organelles driven by liquid-liquid phase separation (LLPS) in a process widely referred to as biomolecular condensation. Some of the best described examples of these structures come from negative stranded RNA viruses, where micrometer size VFs are formed toward the end of the infectious cycle. We here discuss some basic principles of LLPS in connection with several examples of VFs and propose a view, which integrates viral replication mechanisms with the biochemistry underlying liquid-like organelles. In this view, viral protein and RNA components gradually accumulate up to a critical point during infection where phase separation is triggered. This yields an increase in transcription that leads in turn to increased translation and a consequent growth of initially formed condensates. According to chemical principles behind phase separation, an increase in the concentration of components increases the size of the condensate. A positive feedback cycle would thus generate in which crucial components, in particular nucleoproteins and viral polymerases, reach their highest levels required for genome replication. Progress in understanding viral biomolecular condensation leads to exploration of novel therapeutics. Furthermore, it provides insights into the fundamentals of phase separation in the regulation of cellular gene function given that virus replication and transcription, in particular those requiring host polymerases, are governed by the same biochemical principles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

