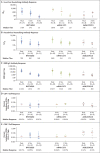
Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines
- PMID: 34648703
- PMCID: PMC8531985
- DOI: 10.1056/NEJMc2115596
Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
