Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa
- PMID: 34648733
- PMCID: PMC8799632
- DOI: 10.1016/S1473-3099(21)00453-9
Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa
Erratum in
-
Correction to Lancet Infect Dis 2022; 22: 284-94.Lancet Infect Dis. 2022 Feb;22(2):e41. doi: 10.1016/S1473-3099(22)00010-X. Lancet Infect Dis. 2022. PMID: 35092804 Free PMC article. No abstract available.
Abstract
Background: Expanding outbreaks of circulating vaccine-derived type 2 poliovirus (cVDPV2) across Africa after the global withdrawal of trivalent oral poliovirus vaccine (OPV) in 2016 are delaying global polio eradication. We aimed to assess the effect of outbreak response campaigns with monovalent type 2 OPV (mOPV2) and the addition of inactivated poliovirus vaccine (IPV) to routine immunisation.
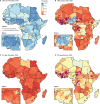
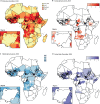
Methods: We used vaccination history data from children under 5 years old with non-polio acute flaccid paralysis from a routine surveillance database (the Polio Information System) and setting-specific OPV immunogenicity data from the literature to estimate OPV-induced and IPV-induced population immunity against type 2 poliomyelitis between Jan 1, 2015, and June 30, 2020, for 51 countries in Africa. We investigated risk factors for reported cVDPV2 poliomyelitis including population immunity, outbreak response activities, and correlates of poliovirus transmission using logistic regression. We used the model to estimate cVDPV2 risk for each 6-month period between Jan 1, 2016, and June 30, 2020, with different numbers of mOPV2 campaigns and compared the timing and location of actual mOPV2 campaigns and the number of mOPV2 campaigns required to reduce cVDPV2 risk to low levels.
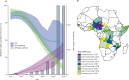
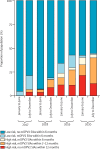
Findings: Type 2 OPV immunity among children under 5 years declined from a median of 87% (IQR 81-93) in January-June, 2016 to 14% (9-37) in January-June, 2020. Type 2 immunity from IPV among children under 5 years increased from 3% (<1-6%) in January-June, 2016 to 35% (24-47) in January-June, 2020. The probability of cVDPV2 poliomyelitis among children under 5 years was negatively correlated with OPV-induced and IPV-induced immunity and mOPV2 campaigns (adjusted odds ratio: OPV 0·68 [95% CrI 0·60-0·76], IPV 0·82 [0·68-0·99] per 10% absolute increase in estimated population immunity, mOPV2 0·30 [0·20-0·44] per campaign). Vaccination campaigns in response to cVDPV2 outbreaks have been smaller and slower than our model shows would be necessary to reduce risk to low levels, covering only 11% of children under 5 years who are predicted to be at risk within 6 months and only 56% within 12 months.
Interpretation: Our findings suggest that as mucosal immunity declines, larger or faster responses with vaccination campaigns using type 2-containing OPV will be required to stop cVDPV2 transmission. IPV-induced immunity also has an important role in reducing the burden of cVDPV2 poliomyelitis in Africa.
Funding: Bill & Melinda Gates Foundation, Medical Research Council Centre for Global Infectious Disease Analysis, and WHO.
Translation: For the French translation of the abstract see Supplementary Materials section.
© 2022 World Health Organization; licensee Elsevier. This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
References
-
- WHO Polio information system. https://extranet.who.int/polis/public/CaseCount.aspx
-
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:934–938. - PubMed
-
- Thompson KM, Duintjer Tebbens RJ. Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis. 2014;210(suppl 1):S475–S484. - PubMed
-
- Global Polio Eradication Initiative Responding to a poliovirus event and outbreak: protocol for poliovirus type 2 standard operating procedures. 2017. https://polioeradication.org/wp-content/uploads/2018/01/pol-sop-respondi...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

