Regression discontinuity analysis for pharmacovigilance: statin example reflected trial findings showing little evidence of harm
- PMID: 34648942
- PMCID: PMC8982642
- DOI: 10.1016/j.jclinepi.2021.10.003
Regression discontinuity analysis for pharmacovigilance: statin example reflected trial findings showing little evidence of harm
Abstract
Objectives: The study aims to explore the use of regression discontinuity analysis (RDA) to examine effects of prescription of statins on total cholesterol and adverse outcomes (type 2 diabetes, rhabdomyolysis and myopathy, myalgia and myositis, liver disease, CVD, and mortality).
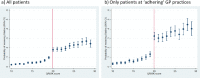
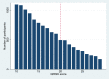
Study design and setting: We conducted a prospective cohort study using the Clinical Practice Research Datalink including patients with QRISK scores of 10 to 30 in 2010 to 2013 who were last followed-up in October 2016. Comparing patients with QRISK≥20 and QRISK<20, we explored RDA assumptions, provided proof of concept analyses (total cholesterol as outcome), and investigated the effect of statins prescription on adverse outcomes.
Result: RDA confirmed statin prescription reduced total cholesterol (Mean difference (MD) -1.33 mmol/L, 95%Confidence Interval (CI) -1.93 to -0.73). RDA provided little evidence for adverse effects on diabetes, myalgia and myositis, liver disease, CVD, or mortality. The RDA analysis findings are similar to RCT results. Findings from non-RDA analysis agree with published observational studies.
Conclusion: RDA can be used with large routine clinical datasets to provide evidence on effects of medications which are prescribed according to a threshold. Testable RDA assumptions were satisfied, but confidence intervals were wide, partly due to the low compliance with the prescribing threshold.
Keywords: Cardiovascular disease; Epidemiology; Health services research; QRISK score; Regression discontinuity analysis; Statins.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Shadish WR, Cook TD, DT C. Houghton Mifflin company; New York: 2002. Experimental and quasi-experimental designs for generalized causal inference.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

