Impact of Joint Lung Cancer Screening and Cessation Interventions Under the New Recommendations of the U.S. Preventive Services Task Force
- PMID: 34648947
- PMCID: PMC8692396
- DOI: 10.1016/j.jtho.2021.09.011
Impact of Joint Lung Cancer Screening and Cessation Interventions Under the New Recommendations of the U.S. Preventive Services Task Force
Abstract
Introduction: In 2021, the U.S. Preventive Services Task Force (USPSTF) revised its lung cancer screening recommendations expanding its eligibility. As more smokers become eligible, cessation interventions at the point of screening could enhance the benefits. Here, we evaluate the effects of joint screening and cessation interventions under the new recommendations.
Methods: A validated lung cancer natural history model was used to estimate lifetime number of low-dose computed tomography screens, percentage ever screened, lung cancer deaths, lung cancer deaths averted, and life-years gained for the 1960 U.S. birth cohort aged 45 to 90 years (4.5 million individuals). Screening occurred according to the USPSTF 2013 and 2021 recommendations with varying uptake (0%, 30%, 100%), with or without a cessation intervention at the point of screening with varying effectiveness (15%, 100%).
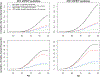
Results: Screening 30% of the eligible population according to the 2021 criteria with no cessation intervention (USPSTF 2021, 30% uptake, without cessation intervention) was estimated to result in 6845 lung cancer deaths averted and 103,725 life-years gained. These represent 28% and 34% increases, respectively, relative to screening according to the 2013 guidelines (USPSTF 2013, 30% uptake, without cessation intervention). Adding a cessation intervention at the time of the first screen with 15% effectiveness (USPSTF 2021, 30% uptake, with cessation intervention with 15% effectiveness) was estimated to result in 2422 additional lung cancer deaths averted (9267 total, ∼73% increase versus USPSTF 2013, 30% uptake, without cessation intervention) and 322,785 life-years gained (∼318% increase). Screening 100% of the eligible according to the 2021 guidelines with no cessation intervention (USPSTF 2021, 100% uptake, without cessation intervention) was estimated to result in 23,444 lung cancer deaths averted (∼337% increase versus USPSTF 2013, 30% uptake, without cessation intervention) and 354,330 life-years gained (∼359% increase). Adding a cessation intervention with 15% effectiveness (USPSTF 2021, 100% uptake, with cessation intervention with 15% effectiveness) would result in 31,998 lung cancer deaths averted (∼497% increase versus USPSTF 2013, 30% uptake, without cessation intervention) and 1,086,840 life-years gained (∼1309% increase).
Conclusions: Joint screening and cessation interventions would result in considerable lung cancer deaths averted and life-years gained. Adding a one-time cessation intervention of modest effectiveness (15%) results in comparable life-years gained as increasing screening uptake from 30% to 100% because while cessation decreases mortality from many causes, screening only reduces lung cancer mortality. This simulation indicates that incorporating cessation programs into screening practice should be a priority as it can maximize overall benefits.
Keywords: CISNET; Cesssation interventions within lung screening; Deaths averted; Life-years gained; Lung cancer screening; Simulation modeling.
Copyright © 2021 International Association for the Study of Lung Cancer. All rights reserved.
Conflict of interest statement
The authors report not conflict of interests.
Figures

Comment in
-
Expansion of Guideline-Recommended Lung Cancer Screening Eligibility: Implications for Health Equity of Joint Screening and Cessation Interventions.J Thorac Oncol. 2022 Jan;17(1):13-15. doi: 10.1016/j.jtho.2021.10.005. J Thorac Oncol. 2022. PMID: 34930605 No abstract available.
References
-
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. Jama. 2021;325(10):962–970. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

