Sulourea-coordinated Pd nanocubes for NIR-responsive photothermal/H2S therapy of cancer
- PMID: 34649589
- PMCID: PMC8515682
- DOI: 10.1186/s12951-021-01042-9
Sulourea-coordinated Pd nanocubes for NIR-responsive photothermal/H2S therapy of cancer
Abstract
Background: Photothermal therapy (PTT) frequently cause thermal resistance in tumor cells by inducing the heat shock response, limiting its therapeutic effect. Hydrogen sulfide (H2S) with appropriate concentration can reverse the Warburg effect in cancer cells. The combination of PTT with H2S gas therapy is expected to achieve synergistic tumor treatment.
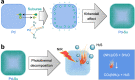
Methods: Here, sulourea (Su) is developed as a thermosensitive/hydrolysable H2S donor to be loaded into Pd nanocubes through in-depth coordination for construction of the Pd-Su nanomedicine for the first time to achieve photo-controlled H2S release, realizing the effective combination of photothermal therapy and H2S gas therapy.
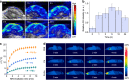
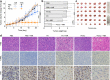
Results: The Pd-Su nanomedicine shows a high Su loading capacity (85 mg g-1), a high near-infrared (NIR) photothermal conversion efficiency (69.4%), and NIR-controlled H2S release by the photothermal-triggered hydrolysis of Su. The combination of photothermal heating and H2S produces a strong synergetic effect by H2S-induced inhibition of heat shock response, thereby effectively inhibiting tumor growth. Moreover, high intratumoral accumulation of the Pd-Su nanomedicine after intravenous injection also enables photothermal/photoacoustic dual-mode imaging-guided tumor treatment.
Conclusions: The proposed NIR-responsive heat/H2S release strategy provides a new approach for effective cancer therapy.
Keywords: Gas therapy; Hydrogen sulfide; Nanomedicine; Pd nanocubes; Photothermal therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- 51872188/national natural science foundation of china
- 20180309154519685/special funds for the development of strategic emerging industries in shenzhen
- 860-00000210/szu top ranking project
- 20ZR1441400/natural science foundation of shanghai
- D18020/"111" innovation and talent recruitment base on photochemical and energy materials
LinkOut - more resources
Full Text Sources
Miscellaneous

