Beyond cuts and scrapes: plasmin in malaria and other vector-borne diseases
- PMID: 34649773
- PMCID: PMC8758534
- DOI: 10.1016/j.pt.2021.09.008
Beyond cuts and scrapes: plasmin in malaria and other vector-borne diseases
Abstract
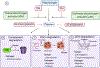
Plasmodium and other vector-borne pathogens have evolved mechanisms to hijack the mammalian fibrinolytic system to facilitate infection of the human host and the invertebrate vector. Plasmin, the effector protease of fibrinolysis, maintains homeostasis in the blood vasculature by degrading the fibrin that forms blood clots. Plasmin also degrades proteins from extracellular matrices, the complement system, and immunoglobulins. Here, we review some of the mechanisms by which vector-borne pathogens interact with components of the fibrinolytic system and co-opt its functions to facilitate transmission and infection in the host and the vector. Further, we discuss innovative strategies beyond conventional therapeutics that could be developed to target the interaction of vector-borne pathogens with the fibrinolytic proteins and prevent their transmission.
Keywords: fibrinolysis; malaria; plasmin; vector-borne diseases.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
References
-
- Sperandio B, et al. (2015) Mucosal physical and chemical innate barriers: Lessons from microbial evasion strategies. Semin Immunol 27, 111–118 - PubMed
-
- Finlay BB and McFadden G (2006) Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell 124, 767–782 - PubMed
-
- Arca B and Ribeiro JM (2018) Saliva of hematophagous insects: a multifaceted toolkit. Curr Opin Insect Sci 29, 102–109 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

